Encoding by synchronization in the primate striatum
- PMID: 23486956
- PMCID: PMC6619016
- DOI: 10.1523/JNEUROSCI.4791-12.2013
Encoding by synchronization in the primate striatum
Abstract
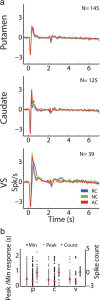
Information is encoded in the nervous system through the discharge and synchronization of single neurons. The striatum, the input stage of the basal ganglia, is divided into three territories: the putamen, the caudate, and the ventral striatum, all of which converge onto the same motor pathway. This parallel organization suggests that there are multiple and competing systems in the basal ganglia network controlling behavior. To explore which mechanism(s) enables the different striatal domains to encode behavioral events and to control behavior, we compared the neural activity of phasically active neurons [medium spiny neurons (MSNs), presumed projection neurons] and tonically active neurons (presumed cholinergic interneurons) across striatal territories from monkeys during the performance of a well practiced task. Although neurons in all striatal territories displayed similar spontaneous discharge properties and similar temporal modulations of their discharge rates to the behavioral events, their correlation structure was profoundly different. The distributions of signal and noise correlation of pairs of putamen MSNs were strongly shifted toward positive correlations and these two measures were correlated. In contrast, MSN pairs in the caudate and ventral striatum displayed symmetrical, near-zero signal and noise correlation distributions. Furthermore, only putamen MSN pairs displayed different noise correlation dynamics to rewarding versus neutral/aversive cues. Similarly, the noise correlation between tonically active neuron pairs was stronger in the putamen than in the caudate. We suggest that the level of synchronization of the neuronal activity and its temporal dynamics differentiate the striatal territories and may thus account for the different roles that striatal domains play in behavioral control.
Figures
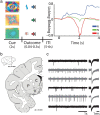
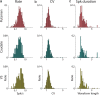

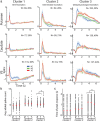
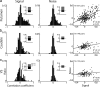


References
-
- Abeles M. Corticonics: neural circuits of the cerebral cortex. Cambridge: Cambridge UP; 1991.
-
- Adler A, Joshua M, Rivlin-Etzion M, Mitelman R, Marmor O, Prut Y, Bergman H. Neurons in both pallidal segments change their firing properties similarly prior to closure of the eyes. J Neurophysiol. 2010;103:346–359. - PubMed
-
- Aertsen AM, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.”. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992;257:1412–1415. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
