Single and multiple injections of subconjunctival ranibizumab for early, recurrent pterygium
- PMID: 23486999
- PMCID: PMC3592556
- DOI: 10.2147/OPTH.S40400
Single and multiple injections of subconjunctival ranibizumab for early, recurrent pterygium
Abstract
Purpose: To assess the effect of single versus multiple subconjunctival ranibizumab injections in patients with an early pterygium recurrence.
Setting: Single-center, academic practice.
Study population: Nine patients with early pterygium recurrence.
Observational procedure: Subconjunctival ranibizumab (0.5 mg/0.05 mL) was administered adjacent to pterygium recurrence. Group 1 (n = 5) received one injection; group 2 (n = 4) received three injections (time points 0, 2, and 4 weeks) with the ability to retreat as needed.
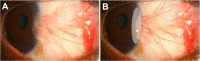
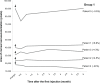
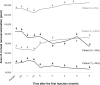
Main outcome measures: Effect of ranibizumab on conjunctival hyperemia and corneal neovascular area over a 6-month follow-up period.
Results: In the single injection group, a decrease in conjunctival hyperemia was noted in all patients on postinjection day 1. At follow up, hyperemia grade fluctuated, although all patients had less hyperemia than at baseline. In the recurrent injection group, the median number of injections was 8.5 (range 7 to 9) over the 6 months. In spite of the repeated injections, the pattern of conjunctival hyperemia was similar to that of the single injection group. In group 1, corneal neovascularization remained relatively unchanged over the 6-month period in four patients and decreased in one patient by 24%. In group 2, corneal neovascularization increased in one patient by 39%, remained stable in one patient, and decreased in two patients by 34% and 44%.
Conclusion: This is the first study to evaluate the role of ranibizumab in the treatment of an early pterygium recurrence and the first to compare multiple versus single injections. Recurrent injections did not appear to be superior to a single injection with regards to conjunctival hyperemia.
Keywords: conjunctival hyperemia; corneal neovascularization; pterygium; ranibizumab; recurrence.
Figures


References
-
- Bock F, König Y, Kruse F, Baier M, Cursiefen C. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):281–284. - PubMed
-
- DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007;125(6):834–836. - PubMed
-
- Doctor PP, Bhat PV, Foster CS. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008;27(9):992–995. - PubMed
-
- Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A. Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr Eye Res. 2008;33(1):23–28. - PubMed
-
- Besharati MR, Manaviat MR, Souzani A. Subconjunctival bevacizumab injection in treatment of pterygium. Acta Med Iran. 2011;49(3):179–183. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

