Activation of conventional kinesin motors in clusters by Shaw voltage-gated K+ channels
- PMID: 23487040
- PMCID: PMC3666255
- DOI: 10.1242/jcs.122234
Activation of conventional kinesin motors in clusters by Shaw voltage-gated K+ channels
Abstract
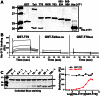
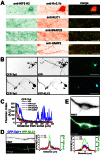
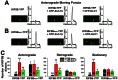
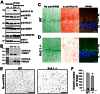
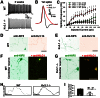
The conventional kinesin motor transports many different cargos to specific locations in neurons. How cargos regulate motor function remains unclear. Here we focus on KIF5, the heavy chain of conventional kinesin, and report that the Kv3 (Shaw) voltage-gated K(+) channel, the only known tetrameric KIF5-binding protein, clusters and activates KIF5 motors during axonal transport. Endogenous KIF5 often forms clusters along axons, suggesting a potential role of KIF5-binding proteins. Our biochemical assays reveal that the high-affinity multimeric binding between the Kv3.1 T1 domain and KIF5B requires three basic residues in the KIF5B tail. Kv3.1 T1 competes with the motor domain and microtubules, but not with kinesin light chain 1 (KLC1), for binding to the KIF5B tail. Live-cell imaging assays show that four KIF5-binding proteins, Kv3.1, KLC1 and two synaptic proteins SNAP25 and VAMP2, differ in how they regulate KIF5B distribution. Only Kv3.1 markedly increases the frequency and number of KIF5B-YFP anterograde puncta. Deletion of Kv3.1 channels reduces KIF5 clusters in mouse cerebellar neurons. Therefore, clustering and activation of KIF5 motors by Kv3 regulate the motor number in carrier vesicles containing the channel proteins, contributing not only to the specificity of Kv3 channel transport, but also to the cargo-mediated regulation of motor function.
Keywords: Axonal transport; Cargo-motor interaction; Kinesin light chain; Molecular motor; Synaptic protein; Voltage-gated potassium channel.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

