Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline
- PMID: 23487317
- PMCID: PMC3621486
- DOI: 10.1242/dev.090662
Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline
Abstract
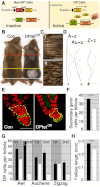
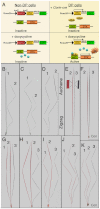
Although the hair shaft is derived from the progeny of keratinocyte stem cells in the follicular epithelium, the growth and differentiation of follicular keratinocytes is guided by a specialized mesenchymal population, the dermal papilla (DP), that is embedded in the hair bulb. Here we show that the number of DP cells in the follicle correlates with the size and shape of the hair produced in the mouse pelage. The same stem cell pool gives rise to hairs of different sizes or types in successive hair cycles, and this shift is accompanied by a corresponding change in DP cell number. Using a mouse model that allows selective ablation of DP cells in vivo, we show that DP cell number dictates the size and shape of the hair. Furthermore, we confirm the hypothesis that the DP plays a crucial role in activating stem cells to initiate the formation of a new hair shaft. When DP cell number falls below a critical threshold, hair follicles with a normal keratinocyte compartment fail to generate new hairs. However, neighbouring follicles with a few more DP cells can re-enter the growth phase, and those that do exploit an intrinsic mechanism to restore both DP cell number and normal hair growth. These results demonstrate that the mesenchymal niche directs stem and progenitor cell behaviour to initiate regeneration and specify hair morphology. Degeneration of the DP population in mice leads to the types of hair thinning and loss observed during human aging, and the results reported here suggest novel approaches to reversing hair loss.
Figures



References
-
- Alcaraz M. V., Villena A., Pérez de Vargas I. (1993). Quantitative study of the human hair follicle in normal scalp and androgenetic alopecia. J. Cutan. Pathol. 20, 344–349 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

