Systematic analysis of enhancer and critical cis-acting RNA elements in the protein-encoding region of the hepatitis C virus genome
- PMID: 23487449
- PMCID: PMC3648135
- DOI: 10.1128/JVI.00840-12
Systematic analysis of enhancer and critical cis-acting RNA elements in the protein-encoding region of the hepatitis C virus genome
Abstract
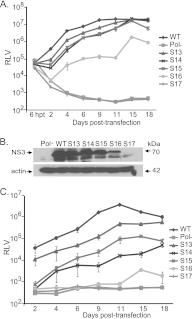
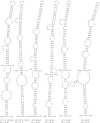
Hepatitis C virus (HCV) causes chronic hepatitis, cirrhosis, and liver cancer. cis-acting RNA elements of the HCV genome are critical for translation initiation and replication of the viral genome. We hypothesized that the coding regions of nonstructural proteins harbor enhancer and essential cis-acting replication elements (CRE). In order to experimentally identify new cis RNA elements, we utilized an unbiased approach to introduce synonymous substitutions. The HCV genome coding for nonstructural proteins (nucleotide positions 3872 to 9097) was divided into 17 contiguous segments. The wobble nucleotide positions of each codon were replaced, resulting in 33% to 41% nucleotide changes. The HCV genome containing one of each of 17 mutant segments (S1 to S17) was tested for genome replication and infectivity. We observed that silent mutations in segment 13 (S13) (nucleotides [nt] 7457 to 7786), S14 (nt 7787 to 8113), S15 (nt 8114 to 8440), S16 (nt 8441 to 8767), and S17 (nt 8768 to 9097) resulted in impaired genome replication, suggesting CRE structures are enriched in the NS5B region. Subsequent high-resolution mutational analysis of NS5B (nt 7787 to 9289) using approximately 51-nucleotide contiguous subsegment mutant viruses having synonymous mutations revealed that subsegments SS8195-8245, SS8654-8704, and SS9011-9061 were required for efficient viral growth, suggesting that these regions act as enhancer elements. Covariant nucleotide substitution analysis of a stem-loop, JFH-SL9098, revealed the formation of an extended stem structure, which we designated JFH-SL9074. We have identified new enhancer RNA elements and an extended stem-loop in the NS5B coding region. Genetic modification of enhancer RNA elements can be utilized for designing attenuated HCV vaccine candidates.
Figures







References
-
- Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. 2006. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144:705–714 - PubMed
-
- Alter MJ. 1997. Epidemiology of hepatitis C. Hepatology 26:62S–65S - PubMed
-
- Wasley A, Grytdal S, Gallagher K. 2008. Surveillance for acute viral hepatitis—United States, 2006. MMWR Surveill Summ. 57:1–24 - PubMed
-
- Perz JF, Alter MJ. 2006. The coming wave of HCV-related liver disease: dilemmas and challenges. J. Hepatol. 44:441–443 - PubMed
-
- El-Serag HB. 2002. Hepatocellular carcinoma: an epidemiologic view. J. Clin. Gastroenterol. 35:S72–S78 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

