Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa)
- PMID: 23487517
- PMCID: PMC3659305
- DOI: 10.1093/eurheartj/eht039
Betrixaban compared with warfarin in patients with atrial fibrillation: results of a phase 2, randomized, dose-ranging study (Explore-Xa)
Abstract
Aims: Patients with atrial fibrillation (AF) are at increased risk of stroke. Betrixaban is a novel oral factor Xa inhibitor administered once daily, mostly excreted unchanged in the bile and with low (17%) renal excretion.
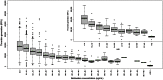
Methods and results: Patients with AF and more than one risk factor for stroke were randomized to one of three blinded doses of betrixaban (40, 60, or 80 mg once daily) or unblinded warfarin, adjusted to an international normalized ratio of 2.0-3.0. The primary outcome was major or clinically relevant non-major bleeding. The mean follow-up was 147 days. Among 508 patients randomized, the mean CHADS2 score was 2.2; 87% of patients had previously received vitamin K antagonist therapy. The time in therapeutic range on warfarin was 63.4%. There were one, five, five, and seven patients with a primary outcome on betrixaban 40, 60, 80 mg daily, or warfarin, respectively. The rate of the primary outcome was lowest on betrixaban 40 mg (hazard ratio compared with warfarin = 0.14, exact stratified log-rank P-value 0.04, unadjusted for multiple testing). Rates of the primary outcome with betrixaban 60 or 80 mg were more similar to those of wafarin. Two ischaemic strokes occurred, one each on betrixaban 60 and 80 mg daily. There were two vascular deaths, one each on betrixaban 40 mg and warfarin. Betrixaban was associated with higher rates of diarrhoea than warfarin.
Conclusion: Betrixaban was well tolerated and had similar or lower rates of bleeding compared with well-controlled warfarin in patients with AF at risk for stroke.
Keywords: Atrial fibrillation; Betrixaban; Stroke.
Figures

References
-
- Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, McGregor VK, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL ACC/AHA Task Force Members. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. ESC Committee for Practice Guidelines doi:10.1161/CIRCULATIONAHA.106.177292. - DOI - PubMed
-
- Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-based Clinical Practice Guidelines (8th edition) Chest. 2008;133(6 Suppl.):160S–198S. doi:10.1378/chest.08-0670. - DOI - PubMed
-
- Petersen P, Boysen G, Godtfredsen J, Andersen ED, Andersen B. Placebo-controlled, randomised trial of warfarin and aspirin for prevention of thromboembolic complications in chronic atrial fibrillation. The Copenhagen AFASAK Study. Lancet. 1989;1:175–179. PubMed PMID: 2563096 doi:10.1016/S0140-6736(89)91200-2. - DOI - PubMed
-
- Stroke Prevention in Atrial Fibrillation Investigators. Warfarin versus aspirin for prevention of thromboembolism in atrial fibrillation: Stroke Prevention in Atrial Fibrillation II Study. Lancet. 1994;343:687–691. - PubMed
-
- Boston Area Anticoagulation Trial for Atrial Fibrillation (BAATAF) Investigators. The effect of low-dose warfarin on the risk of stroke in patients with nonrheumatic atrial fibrillation. N Engl J Med. 1990;323:1505–1511. doi:10.1056/NEJM199011293232201. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

