A biologically plausible embodied model of action discovery
- PMID: 23487577
- PMCID: PMC3594743
- DOI: 10.3389/fnbot.2013.00004
A biologically plausible embodied model of action discovery
Abstract
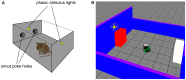
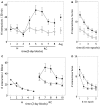
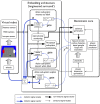
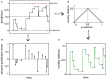
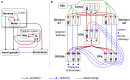
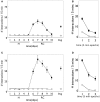
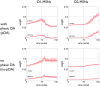
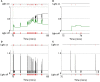
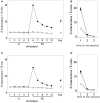
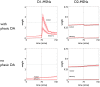
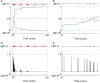
During development, animals can spontaneously discover action-outcome pairings enabling subsequent achievement of their goals. We present a biologically plausible embodied model addressing key aspects of this process. The biomimetic model core comprises the basal ganglia and its loops through cortex and thalamus. We incorporate reinforcement learning (RL) with phasic dopamine supplying a sensory prediction error, signalling "surprising" outcomes. Phasic dopamine is used in a cortico-striatal learning rule which is consistent with recent data. We also hypothesized that objects associated with surprising outcomes acquire "novelty salience" contingent on the predicability of the outcome. To test this idea we used a simple model of prediction governing the dynamics of novelty salience and phasic dopamine. The task of the virtual robotic agent mimicked an in vivo counterpart (Gancarz et al., 2011) and involved interaction with a target object which caused a light flash, or a control object which did not. Learning took place according to two schedules. In one, the phasic outcome was delivered after interaction with the target in an unpredictable way which emulated the in vivo protocol. Without novelty salience, the model was unable to account for the experimental data. In the other schedule, the phasic outcome was reliably delivered and the agent showed a rapid increase in the number of interactions with the target which then decreased over subsequent sessions. We argue this is precisely the kind of change in behavior required to repeatedly present representations of context, action and outcome, to neural networks responsible for learning action-outcome contingency. The model also showed cortico-striatal plasticity consistent with learning a new action in basal ganglia. We conclude that action learning is underpinned by a complex interplay of plasticity and stimulus salience, and that our model contains many of the elements for biological action discovery to take place.
Keywords: action selection; basal ganglia; intrinsic motivation; operant behavior; phasic dopamine; reinforcement learning; synaptic plasticity.
Figures



References
-
- Alexander W. H., Sporns O. (2002). An embodied model of learning, plasticity, and reward. Adaptive Behav. 10, 143–159
-
- Barto A. G. (1995). Adaptive critics and the basal ganglia, in Models of Information Processing in the Basal Ganglia, eds Houk J. C., Davis J., Beiser D. (Cambridge, MA: MIT Press; ), 215–232
-
- Barto A. G., Singh S., Chantanez N. (2004). Intrinsically motivated reinforcement learning, in 18th Annual Conference on Neural Information Processing Systems (NIPS) (Vancouver, BC: ).
LinkOut - more resources
Full Text Sources
Other Literature Sources

