Assessment of the quality of clinical practice guidelines in Korea using the AGREE Instrument
- PMID: 23487579
- PMCID: PMC3594597
- DOI: 10.3346/jkms.2013.28.3.357
Assessment of the quality of clinical practice guidelines in Korea using the AGREE Instrument
Abstract
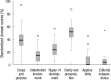
The objective of this study was to conduct the systematic evaluation of methodological quality of clinical practice guidelines (CPGs) in Korea. The authors conducted a very comprehensive literature search to identify potential CPGs for evaluation. CPGs were selected which were consistent with a predetermined criteria. Four reviewers evaluated the quality of the CPGs using the Appraisal of Guidelines, Research and Evaluation (AGREE) Instrument. AGREE item scores and standardized domain scores were calculated. The inter-rater reliability of each domain was evaluated using the intra-class correlation coefficient (ICC). Consequently, 66 CPGs were selected and their quality evaluated. ICCs for CPG appraisal using the AGREE Instrument ranged from 0.626 to 0.877. Except for the "Scope and Purpose" and "Clarity and Presentation domains", 80% of CPGs scored less than 40 in all other domains. This review shows that many Korean research groups and academic societies have made considerable efforts to develop CPGs, and the number of CPGs has increased over time. However, the quality of CPGs in Korea were not good according to the AGREE Instrument evaluation. Therefore, we should make more of an effort to ensure the high quality of CPGs.
Keywords: Clinical Practice Guideline; Quality Improvement.
Figures

References
-
- Field MJ, Lohr KN. Clinical practice guidelines: directions for a new program. Washington, D.C.: National Academy Press; 1990. - PubMed
-
- Pauly MV, Eisenberg JM, Radany MH, Erder MH, Feldman R, Schwartz JS. Paying physicians: options for controlling cost, volume, and intensity of services. Ann Arbor: Health Administration Press; 1992.
-
- Burgers JS, Grol R, Klazinga NS, Mäkelä M, Zaat J AGREE Collaboration. Towards evidence-based clinical practice: an international survey of 18 clinical guideline programs. Int J Qual Health Care. 2003;15:31–45. - PubMed
-
- Nagy E, Watine J, Bunting PS, Onody R, Oosterhuis WP, Rogic D, Sandberg S, Boda K, Horvath AR IFCC Task Force on the Global Campaign for Diabetes Mellitus. Do guidelines for the diagnosis and monitoring of diabetes mellitus fulfill the criteria of evidence-based guideline development? Clin Chem. 2008;54:1872–1882. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

