Mechanics and Function of the Pulmonary Vasculature: Implications for Pulmonary Vascular Disease and Right Ventricular Function
- PMID: 23487595
- PMCID: PMC3593606
- DOI: 10.1002/cphy.c100070
Mechanics and Function of the Pulmonary Vasculature: Implications for Pulmonary Vascular Disease and Right Ventricular Function
Abstract
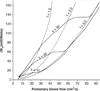
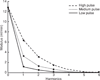
The relationship between cardiac function and the afterload against which the heart muscle must work to circulate blood throughout the pulmonary circulation is defined by a complex interaction between many coupled system parameters. These parameters range broadly and incorporate system effects originating primarily from three distinct locations: input power from the heart, hydraulic impedance from the large conduit pulmonary arteries, and hydraulic resistance from the more distal microcirculation. These organ systems are not independent, but rather, form a coupled system in which a change to any individual parameter affects all other system parameters. The result is a highly nonlinear system which requires not only detailed study of each specific component and the effect of disease on their specific function, but also requires study of the interconnected relationship between the microcirculation, the conduit arteries, and the heart in response to age and disease. Here, we investigate systems-level changes associated with pulmonary hypertensive disease progression in an effort to better understand this coupled relationship.
Figures














References
-
- Alexander RS. The influence of constrictor drugs on the distensibility of the splanchnic venous system, analyzed on the basis of an aortic model. Circ Res. 1954;2:140–147. - PubMed
-
- Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O’Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 1983;68:50–58. - PubMed
-
- Badesch DB, Champion HC, Sanchez MA, Hoeper MM, Loyd JE, Manes A, McGoon M, Naeije R, Olschewski H, Oudiz RJ, Torbicki A. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54:S55–S66. - PubMed
-
- Balzer DT, Kort HW, Day RW, Corneli HM, Kovalchin JP, Cannon BC, Kaine SF, Ivy DD, Webber SA, Rothman A, Ross RD, Aggarwal S, Takahashi M, Waldman JD. Inhaled nitric oxide as a preoperative test (INOP test I): The INOP Test Study Group. Circulation. 2002;106:I76–I81. - PubMed
-
- Barra JG, Armentano RL, Levenson J, Fischer EI, Pichel RH, Simon A. Assessment of smooth muscle contribution to descending thoracic aortic elastic mechanics in conscious dogs. Circ Res. 1993;73:1040–1050. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

