Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1
- PMID: 23487776
- PMCID: PMC3606986
- DOI: 10.1073/pnas.1219777110
Xerocytosis is caused by mutations that alter the kinetics of the mechanosensitive channel PIEZO1
Abstract
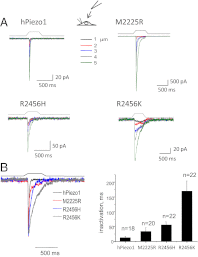
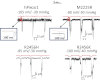
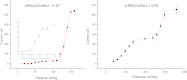
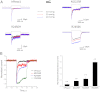
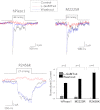
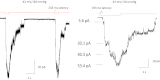
Familial xerocytosis (HX) in humans is an autosomal disease that causes dehydration of red blood cells resulting in hemolytic anemia which has been traced to two individual mutations in the mechanosensitive ion channel, PIEZO1. Each mutation alters channel kinetics in ways that can explain the clinical presentation. Both mutations slowed inactivation and introduced a pronounced latency for activation. A conservative substitution of lysine for arginine (R2456K) eliminated inactivation and also slowed deactivation, indicating that this mutant's loss of charge is not responsible for HX. Fitting the current vs. pressure data to Boltzmann distributions showed that the half-activation pressure, P1/2, for M2225R was similar to that of WT, whereas mutations at position 2456 were left shifted. The absolute stress sensitivity was calibrated by cotransfection and comparison with MscL, a well-characterized mechanosensitive channel from bacteria that is driven by bilayer tension. The slope sensitivity of WT and mutant human PIEZO1 (hPIEZO1) was similar to that of MscL implying that the in-plane area increased markedly, by ∼6-20 nm(2) during opening. In addition to the behavior of individual channels, groups of hPIEZO1 channels could undergo simultaneous changes in kinetics including a loss of inactivation and a long (∼200 ms), silent latency for activation. These observations suggest that hPIEZO1 exists in spatial domains whose global properties can modify channel gating. The mutations that create HX affect cation fluxes in two ways: slow inactivation increases the cation flux, and the latency decreases it. These data provide a direct link between pathology and mechanosensitive channel dysfunction in nonsensory cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



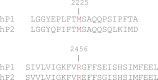
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

