Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response
- PMID: 23487796
- PMCID: PMC3606972
- DOI: 10.1073/pnas.1302265110
Arabidopsis AHP2, AHP3, and AHP5 histidine phosphotransfer proteins function as redundant negative regulators of drought stress response
Abstract
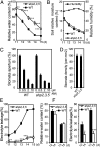
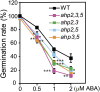
Cytokinin is an essential phytohormone controlling various biological processes, including environmental stress responses. In Arabidopsis, although the cytokinin (CK)-related phosphorelay--consisting of three histidine kinases, five histidine phosphotransfer proteins (AHPs), and a number of response regulators--has been known to be important for stress responses, the AHPs required for CK signaling during drought stress remain elusive. Here, we report that three Arabidopsis AHPs, namely AHP2, AHP3, and AHP5, control responses to drought stress in negative and redundant manner. Loss of function of these three AHP genes resulted in a strong drought-tolerant phenotype that was associated with the stimulation of protective mechanisms. Specifically, cell membrane integrity was improved as well as an increased sensitivity to abscisic acid (ABA) was observed rather than an alteration in ABA-mediated stomatal closure and density. Consistent with their negative regulatory functions, all three AHP genes' expression was down-regulated by dehydration, which most likely resulted from a stress-induced reduction of endogenous CK levels. Furthermore, global transcriptional analysis of ahp2,3,5 leaves revealed down-regulation of many well-known stress- and/or ABA-responsive genes, suggesting that these three AHPs may control drought response in both ABA-dependent and ABA-independent manners. The discovery of mechanisms of activation and the targets of the downstream components of CK signaling involved in stress responses is an important and challenging goal for the study of plant stress regulatory network responses and plant growth. The knowledge gained from this study also has broad potential for biotechnological applications to increase abiotic stress tolerance in plants.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hadiarto T, Tran LS. Progress studies of drought-responsive genes in rice. Plant Cell Rep. 2011;30(3):297–310. - PubMed
-
- Choudhary SP, Yu JQ, Yamaguchi-Shinozaki K, Shinozaki K, Tran LS. Benefits of brassinosteroid crosstalk. Trends Plant Sci. 2012;17(10):594–605. - PubMed
-
- Jogaiah S, Ramsandra Govind S, Tran LS. System biology-based approaches towards understanding drought tolerance in food crops. Crit Rev Biotechnol. 2013;33(1):23–39. - PubMed
-
- Mizuno T. Two-component phosphorelay signal transduction systems in plants: From hormone responses to circadian rhythms. Biosci Biotechnol Biochem. 2005;69(12):2263–2276. - PubMed
-
- Mähönen AP, et al. Cytokinin signaling and its inhibitor AHP6 regulate cell fate during vascular development. Science. 2006;311(5757):94–98. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

