S 50131 and S 51434, two novel small molecule glucokinase activators, lack chronic efficacy despite potent acute antihyperglycaemic activity in diabetic mice
- PMID: 23488540
- PMCID: PMC3696324
- DOI: 10.1111/bph.12172
S 50131 and S 51434, two novel small molecule glucokinase activators, lack chronic efficacy despite potent acute antihyperglycaemic activity in diabetic mice
Abstract
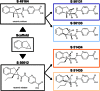
Background and purpose: Small molecule glucokinase activators (GKAs) have been associated with potent antidiabetic efficacy and hepatic steatosis in rodents. This study reports the discovery of S 50131 and S 51434, two novel GKAs with an original scaffold and an atypical pharmacological profile.
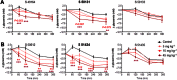
Experimental approach: Activity of the compounds was assessed in vitro by measuring activation of recombinant glucokinase, stimulation of glycogen synthesis in rat hepatocytes and increased insulin secretion from rat pancreatic islets of Langerhans. Efficacy and safety in vivo were evaluated after oral administration in db/db mice by measuring glycaemia, HbA1c and dyslipidaemia-associated events.
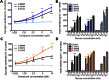
Key results: S 50131 and S 51434 activated GK and stimulated glycogen synthesis in hepatocytes and insulin secretion from pancreatic islets. Unexpectedly, while both compounds effectively lowered glycaemia after acute oral administration, they did not decrease HbA1c after a 4-week treatment in db/db mice. This lack of antidiabetic efficacy was associated with increased plasma free fatty acids (FFAs), contrasting with the effect of GKA50 and N00236460, two GKAs with sustained HbA1c lowering activity but neutral regarding plasma FFAs. S 50131, but not S 51434, also induced hepatic steatosis, as did GKA50 and N00236460. However, a shorter, 4-day treatment resulted in increased hepatic triglycerides without changing the plasma FFA levels, demonstrating dynamic alterations in the lipid profile over time.
Conclusions and implications: In addition to confirming the occurrence of dyslipidaemia with GKAs, these findings provide new insights into understanding how such compounds may sustain or lose efficacy over time.
© 2013 The Authors. British Journal of Pharmacology © 2013 The British Pharmacological Society.
Figures



References
-
- Bebernitz GR, Beaulieu V, Dale BA, Deacon R, Duttaroy A, Gao J, et al. Investigation of functionally liver selective glucokinase activators for the treatment of type 2 diabetes. J Med Chem. 2009;52:6142–6152. - PubMed
-
- Bertrand M, Jackson P, Walther B. Rapid assessment of drug metabolism in the drug discovery process. Eur J Pharm Sci. 2000;11(Suppl. 2):S61–S72. - PubMed
-
- Brocklehurst KJ, Payne VA, Davies RA, Carroll D, Vertigan HL, Wightman HJ, et al. Stimulation of hepatocyte glucose metabolism by novel small molecule glucokinase activators. Diabetes. 2004;53:535–541. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

