Ethanol inhibition of a T-type Ca²+ channel through activity of protein kinase C
- PMID: 23488970
- PMCID: PMC4381783
- DOI: 10.1111/acer.12098
Ethanol inhibition of a T-type Ca²+ channel through activity of protein kinase C
Abstract
Background: T-type calcium channels (T-channels) are widely distributed in the central and peripheral nervous system, where they mediate calcium entry and regulate the intrinsic excitability of neurons. T-channels are dysregulated in response to alcohol administration and withdrawal. We therefore investigated acute ethanol (EtOH) effects and the underlying mechanism of action in human embryonic kidney (HEK) 293 cell lines, as well as effects on native currents recorded from dorsal root ganglion (DRG) neurons cultured from Long-Evans rats.
Methods: Whole-cell voltage-clamp recordings were performed at 32 to 34°C in both HEK cell lines and DRG neurons. The recordings were taken after a 10-minute application of EtOH or protein kinase C (PKC) activator (phorbol 12-myristate 13-acetate [PMA]).
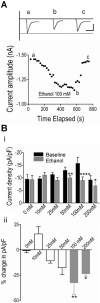
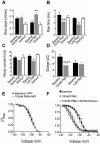
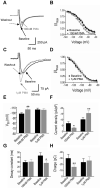
Results: We recorded T-type Ca²⁺ currents (T-currents) from 3 channel isoforms (CaV3.1, CaV3.2, and CaV3.3) before and during administration of EtOH. We found that only 1 isoform, CaV3.2, was significantly affected by EtOH. EtOH reduced current density as well as producing a hyperpolarizing shift in steady-state inactivation of both CaV3.2 currents from HEK 293 cell lines and in native T-currents from DRG neurons that are known to be enriched in CaV3.2. A myristoylated PKC peptide inhibitor (MPI) blocked the major EtOH effects, in both the cell lines and the DRG neurons. However, PMA effects were more complex. Lower concentration PMA (100 nM) replicated the major effects of EtOH, while higher concentration PMA (1 μM) did not, suggesting that the EtOH effects operate through activation of PKC and were mimicked by lower concentration of PMA.
Conclusions: EtOH primarily affects the CaV3.2 isoform of T-type Ca²⁺ channels acting through PKC, highlighting a novel target and mechanism for EtOH effects on excitable membranes.
Keywords: CaV3.2 T-Channel; Dorsal Root Ganglion; Ethanol; Phorbol 12-Myristate 13-Acetate; Protein Kinase C.
Copyright © 2013 by the Research Society on Alcoholism.
Figures




Similar articles
-
Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density.J Physiol. 2006 Dec 1;577(Pt 2):513-23. doi: 10.1113/jphysiol.2006.117440. Epub 2006 Sep 28. J Physiol. 2006. PMID: 17008378 Free PMC article.
-
Melatonin-mediated inhibition of Cav3.2 T-type Ca2+ channels induces sensory neuronal hypoexcitability through the novel protein kinase C-eta isoform.J Pineal Res. 2018 May;64(4):e12476. doi: 10.1111/jpi.12476. Epub 2018 Mar 8. J Pineal Res. 2018. PMID: 29437250
-
Mechanisms of inhibition of T-type calcium current in the reticular thalamic neurons by 1-octanol: implication of the protein kinase C pathway.Mol Pharmacol. 2010 Jan;77(1):87-94. doi: 10.1124/mol.109.059931. Epub 2009 Oct 21. Mol Pharmacol. 2010. PMID: 19846748 Free PMC article.
-
Targeting of CaV3.2 T-type calcium channels in peripheral sensory neurons for the treatment of painful diabetic neuropathy.Pflugers Arch. 2014 Apr;466(4):701-6. doi: 10.1007/s00424-014-1452-z. Epub 2014 Jan 31. Pflugers Arch. 2014. PMID: 24482063 Review.
-
Regulation of the T-type Ca(2+) channel Cav3.2 by hydrogen sulfide: emerging controversies concerning the role of H2 S in nociception.J Physiol. 2016 Aug 1;594(15):4119-29. doi: 10.1113/JP270963. Epub 2016 Feb 25. J Physiol. 2016. PMID: 26804000 Free PMC article. Review.
Cited by
-
Protein kinase C epsilon-mediated modulation of T-type calcium channels underlies alcohol withdrawal hyperexcitability in the midline thalamus.Alcohol Clin Exp Res (Hoboken). 2024 Jul;48(7):1278-1288. doi: 10.1111/acer.15342. Epub 2024 May 13. Alcohol Clin Exp Res (Hoboken). 2024. PMID: 38740544 Free PMC article.
-
Mechanisms and Pharmacotherapy for Ethanol-Responsive Movement Disorders.Front Neurol. 2020 Aug 25;11:892. doi: 10.3389/fneur.2020.00892. eCollection 2020. Front Neurol. 2020. PMID: 32982923 Free PMC article. Review.
-
Ethosuximide reduces electrographical and behavioral correlates of alcohol withdrawal seizure in DBA/2J mice.Alcohol. 2014 Aug;48(5):445-53. doi: 10.1016/j.alcohol.2014.01.010. Epub 2014 May 4. Alcohol. 2014. PMID: 24933286 Free PMC article.
-
Selective Blockade of T-Type Ca2+ Channels is Protective Against Alcohol-Withdrawal Induced Seizure and Mortality.Alcohol Alcohol. 2018 Sep 1;53(5):526-531. doi: 10.1093/alcalc/agy042. Alcohol Alcohol. 2018. PMID: 29912275 Free PMC article.
-
A Role for GABAA Receptor β3 Subunits in Mediating Harmaline Tremor Suppression by Alcohol: Implications for Essential Tremor Therapy.Tremor Other Hyperkinet Mov (N Y). 2024 Apr 26;14:20. doi: 10.5334/tohm.834. eCollection 2024. Tremor Other Hyperkinet Mov (N Y). 2024. PMID: 38681506 Free PMC article.
References
-
- Carbone E, Lux HD. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature. 1984;310:501–502. - PubMed
-
- Chemin J, Mezghrani A, Bidaud I, Dupasquier S, Marger F, C Barrère, Nargeot J, Lory P. Temperature-dependent Modulation of CaV3 T-type Calcium Channels by Protein Kinases C and A in Mammalian Cells. Journal of Biological Chemistry. 2007;282:32710–32718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

