Metabotropic glutamate receptor 5 positive allosteric modulators are neuroprotective in a mouse model of Huntington's disease
- PMID: 23489026
- PMCID: PMC3687670
- DOI: 10.1111/bph.12164
Metabotropic glutamate receptor 5 positive allosteric modulators are neuroprotective in a mouse model of Huntington's disease
Abstract
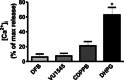
Background and purpose: Huntington's disease (HD) is an autosomal dominant neurodegenerative disorder caused by a polyglutamine expansion in the huntingtin protein. We have previously demonstrated that the cell signalling of the metabotropic glutamate receptor 5 (mGluR5) is altered in a mouse model of HD. Although mGluR5-dependent protective pathways are more activated in HD neurons, intracellular Ca²⁺ release is also more pronounced, which could contribute to excitotoxicity. In the present study, we aim to investigate whether mGluR5 positive allosteric modulators (PAMs) could activate protective pathways without triggering high levels of Ca²⁺ release and be neuroprotective in HD.
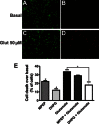
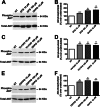
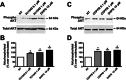
Experimental approach: We performed a neuronal cell death assay to determine which drugs are neuroprotective, Western blot and Ca²⁺ release experiments to investigate the molecular mechanisms involved in this neuroprotection, and object recognition task to determine whether the tested drugs could ameliorate HD memory deficit.
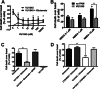
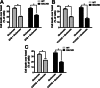
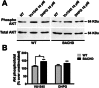
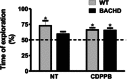
Key results: We find that mGluR5 PAMs can protect striatal neurons from the excitotoxic neuronal cell death promoted by elevated concentrations of glutamate and NMDA. mGluR5 PAMs are capable of activating Akt without triggering increased intracellular Ca²⁺ concentration ([Ca²⁺]i ); and Akt blockage leads to loss of PAM-mediated neuroprotection. Importantly, PAMs' potential as drugs that may be used to treat neurodegenerative diseases is highlighted by the neuroprotection exerted by mGluR5 PAMs on striatal neurons from a mouse model of HD, BACHD. Moreover, mGluR5 PAMs can activate neuroprotective pathways more robustly in BACHD mice and ameliorate HD memory deficit.
Conclusions and implications: mGluR5 PAMs are potential drugs that may be used to treat neurodegenerative diseases, especially HD.
© 2013 The Authors. British Journal of Pharmacology © 2013 The British Pharmacological Society.
Figures

References
-
- Bagetta V, Ghiglieri V, Sgobio C, Calabresi P, Picconi B. Synaptic dysfunction in Parkinson's disease. Biochem Soc Trans. 2010;38:493–497. - PubMed
-
- Baskys A, Bayazitov I, Fang L, Blaabjerg M, Poulsen FR, Zimmer J. Group I metabotropic glutamate receptors reduce excitotoxic injury and may facilitate neurogenesis. Neuropharmacology. 2005;49(Suppl. 1):146–156. - PubMed
-
- Bruno V, Battaglia G, Copani A, Cespedes VM, Galindo MF, Cena V, et al. An activity-dependent switch from facilitation to inhibition in the control of excitotoxicity by group I metabotropic glutamate receptors. Eur J Neurosci. 2001;13:1469–1478. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

