Loss of constitutive activity is correlated with increased thermostability of the human adenosine A2A receptor
- PMID: 23489072
- PMCID: PMC3696323
- DOI: 10.1111/bph.12165
Loss of constitutive activity is correlated with increased thermostability of the human adenosine A2A receptor
Abstract
Background and purpose: Thermostabilization by mutagenesis is one method which has facilitated the determination of high-resolution structures of the adenosine A2A receptor (A(2A)R). Sets of mutations were identified, which both thermostabilized the receptor and resulted in preferential agonist (Rag23 mutant) or antagonist (Rant5 and Rant21) binding forms as assessed by radioligand binding analysis. While the ligand-binding profiles of these mutants are known, the effects these mutations have on receptor activation and downstream signalling are less well characterized.
Experimental approach: Here we have investigated the effects of the thermostabilizing mutations on receptor activation using a yeast cell growth assay. The assay employs an engineered Saccharomyces cerevisiae, MMY24, which couples receptor activation to cell growth.
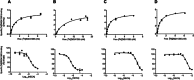
Key results: Analysis of the receptor activation profile revealed that the wild-type (WT) A(2A)R had considerable constitutive activity. In contrast, the Rag23, Rant5 and Rant21 thermostabilized mutants all exhibited no constitutive activity. While the preferentially antagonist-binding mutants Rant5 and Rant21 showed a complete lack of agonist-induced activity, the Rag23 mutant showed high levels of agonist-induced receptor activity. Further analysis using a mutant intermediate between Rag23 and WT indicated that the loss of constitutive activity observed in the agonist responsive mutants was not due to reduced G-protein coupling.
Conclusions and implications: The loss of constitutive activity may be an important feature of these thermostabilized GPCRs. In addition, the constitutively active and agonist-induced active conformations of the A(2A)R are distinct.
© 2013 The Authors. British Journal of Pharmacology © 2013 The British Pharmacological Society.
Figures


Similar articles
-
Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation.Nature. 2011 May 18;474(7352):521-5. doi: 10.1038/nature10136. Nature. 2011. PMID: 21593763 Free PMC article.
-
Arginine 199 and leucine 208 have key roles in the control of adenosine A2A receptor signalling function.PLoS One. 2014 Mar 3;9(3):e89613. doi: 10.1371/journal.pone.0089613. eCollection 2014. PLoS One. 2014. PMID: 24595172 Free PMC article.
-
Thermostabilisation of an agonist-bound conformation of the human adenosine A(2A) receptor.J Mol Biol. 2011 Jun 10;409(3):298-310. doi: 10.1016/j.jmb.2011.03.075. Epub 2011 Apr 9. J Mol Biol. 2011. PMID: 21501622 Free PMC article.
-
Characterization of cancer-related somatic mutations in the adenosine A2B receptor.Eur J Pharmacol. 2020 Aug 5;880:173126. doi: 10.1016/j.ejphar.2020.173126. Epub 2020 Apr 26. Eur J Pharmacol. 2020. PMID: 32348778
-
GPCR structure and activation: an essential role for the first extracellular loop in activating the adenosine A2B receptor.FASEB J. 2011 Feb;25(2):632-43. doi: 10.1096/fj.10-164319. Epub 2010 Oct 28. FASEB J. 2011. PMID: 21030693
Cited by
-
Pharmacological properties of acid N-thiazolylamide FFA2 agonists.Pharmacol Res Perspect. 2015 Jun;3(3):e00141. doi: 10.1002/prp2.141. Epub 2015 May 8. Pharmacol Res Perspect. 2015. PMID: 26236484 Free PMC article.
-
Functional Expression of Adenosine A3 Receptor in Yeast Utilizing a Chimera with the A2AR C-Terminus.Int J Mol Sci. 2020 Jun 26;21(12):4547. doi: 10.3390/ijms21124547. Int J Mol Sci. 2020. PMID: 32604732 Free PMC article.
-
Ligand-induced conformational changes in a SMALP-encapsulated GPCR.Biochim Biophys Acta Biomembr. 2020 Jun 1;1862(6):183235. doi: 10.1016/j.bbamem.2020.183235. Epub 2020 Feb 29. Biochim Biophys Acta Biomembr. 2020. PMID: 32126232 Free PMC article.
-
Experimental and computational analysis of biased agonism on full-length and a C-terminally truncated adenosine A2A receptor.Comput Struct Biotechnol J. 2020 Sep 24;18:2723-2732. doi: 10.1016/j.csbj.2020.09.028. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 33101610 Free PMC article.
-
Recovery of the histamine H3 receptor activity lost in yeast cells through error-prone PCR and in vivo selection.Sci Rep. 2023 Sep 26;13(1):16127. doi: 10.1038/s41598-023-43389-z. Sci Rep. 2023. PMID: 37752220 Free PMC article.
References
-
- Alexander SP, Millns PJ. [(3)H]ZM241385 – an antagonist radioligand for adenosine A(2A) receptors in rat brain. Eur J Pharmacol. 2001;411:205–210. - PubMed
-
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. - PubMed
-
- Dodevski I, Plückthun A. Evolution of three human GPCRs for higher expression and stability. J Mol Biol. 2011;408:599–615. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

