Histamine is required for H₃ receptor-mediated alcohol reward inhibition, but not for alcohol consumption or stimulation
- PMID: 23489295
- PMCID: PMC3764859
- DOI: 10.1111/bph.12170
Histamine is required for H₃ receptor-mediated alcohol reward inhibition, but not for alcohol consumption or stimulation
Abstract
Background and purpose: Conflicting data have been published on whether histamine is inhibitory to the rewarding effects of abused drugs. The purpose of this study was to clarify the role of neuronal histamine and, in particular, H₃ receptors in alcohol dependence-related behaviours, which represent the addictive effects of alcohol.
Experimental approach: Alcohol-induced conditioned place preference (alcohol-CPP) was used to measure alcohol reward. Alcohol-induced locomotor stimulation, alcohol consumption and kinetics were also assessed. mRNA levels were quantified using radioactive in situ hybridization.
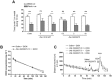
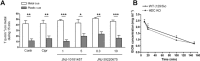
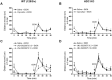
Key results: Low doses of H₃ receptor antagonists, JNJ-10181457 and JNJ-39220675, inhibited alcohol reward in wild-type (WT) mice. However, these H₃ receptor antagonists did not inhibit alcohol reward in histidine decarboxylase knock-out (HDC KO) mice and a lack of histamine did not alter alcohol consumption. Thus H₃ receptor antagonists inhibited alcohol reward in a histamine-dependent manner. Furthermore, WT and HDC KO mice were similarly stimulated by alcohol. The expression levels of dopamine D₁ and D₂ receptors, STEP61 and DARPP-32 mRNA in striatal subregions were unaltered in HDC KO mice. No differences were seen in alcohol kinetics in HDC KO compared to WT control animals. In addition, JNJ-39220675 had no effect on alcohol kinetics in WT mice.
Conclusions and implications: These data suggest that histamine is required for the H₃ receptor-mediated inhibition of alcohol-CPP and support the hypothesis that the brain histaminergic system has an inhibitory role in alcohol reward. Increasing neuronal histamine release via H₃ receptor blockade could therefore be a novel way of treating alcohol dependence.
Keywords: H3 receptor antagonist; alcohol; dopamine; histamine; reward.
© 2013 The Authors. British Journal of Pharmacology © 2013 The British Pharmacological Society.
Figures

Similar articles
-
Histamine H3 receptor antagonist JNJ-39220675 modulates locomotor responses but not place conditioning by dopaminergic drugs.Psychopharmacology (Berl). 2015 Mar;232(6):1143-53. doi: 10.1007/s00213-014-3751-7. Epub 2014 Oct 12. Psychopharmacology (Berl). 2015. PMID: 25308376
-
Histamine and H3 receptor-dependent mechanisms regulate ethanol stimulation and conditioned place preference in mice.Psychopharmacology (Berl). 2010 Jan;208(1):75-86. doi: 10.1007/s00213-009-1710-5. Epub 2009 Nov 13. Psychopharmacology (Berl). 2010. PMID: 19911169
-
Histamine and H3 receptor in alcohol-related behaviors.J Pharmacol Exp Ther. 2011 Jan;336(1):9-16. doi: 10.1124/jpet.110.170928. Epub 2010 Sep 23. J Pharmacol Exp Ther. 2011. PMID: 20864504 Review.
-
Influence of the novel histamine H₃ receptor antagonist ST1283 on voluntary alcohol consumption and ethanol-induced place preference in mice.Psychopharmacology (Berl). 2013 Jul;228(1):85-95. doi: 10.1007/s00213-013-3019-7. Epub 2013 Mar 9. Psychopharmacology (Berl). 2013. PMID: 23474889
-
Modulation of behavior by the histaminergic system: lessons from HDC-, H3R- and H4R-deficient mice.Neurosci Biobehav Rev. 2014 Nov;47:101-21. doi: 10.1016/j.neubiorev.2014.07.020. Epub 2014 Aug 4. Neurosci Biobehav Rev. 2014. PMID: 25102165 Review.
Cited by
-
Histamine pharmacology: four years on.Br J Pharmacol. 2013 Sep;170(1):1-3. doi: 10.1111/bph.12319. Br J Pharmacol. 2013. PMID: 23944741 Free PMC article.
-
Therapeutic Potential of Histamine H3 Receptors in Substance Use Disorders.Curr Top Behav Neurosci. 2022;59:169-191. doi: 10.1007/7854_2022_372. Curr Top Behav Neurosci. 2022. PMID: 35704272 Review.
-
Targeting Histamine and Histamine Receptors for Memory Regulation: An Emotional Perspective.Curr Neuropharmacol. 2024;22(11):1846-1869. doi: 10.2174/1570159X22666240128003108. Curr Neuropharmacol. 2024. PMID: 38288837 Free PMC article. Review.
-
Ethanol Induces Sedation and Hypnosis via Inhibiting Histamine Release in Mice.Neurochem Res. 2019 Jul;44(7):1764-1772. doi: 10.1007/s11064-019-02813-5. Epub 2019 May 15. Neurochem Res. 2019. PMID: 31093904
-
Histamine H3 receptor antagonist JNJ-39220675 modulates locomotor responses but not place conditioning by dopaminergic drugs.Psychopharmacology (Berl). 2015 Mar;232(6):1143-53. doi: 10.1007/s00213-014-3751-7. Epub 2014 Oct 12. Psychopharmacology (Berl). 2015. PMID: 25308376
References
-
- Airaksinen MS, Paetau A, Paljarvi L, Reinikainen K, Riekkinen P, Suomalainen R, et al. Histamine neurons in human hypothalamus: anatomy in normal and Alzheimer diseased brains. Neuroscience. 1991a;44:465–481. - PubMed
-
- Airaksinen MS, Reinikainen K, Riekkinen P, Panula P. Neurofibrillary tangles and histamine-containing neurons in Alzheimer hypothalamus. Agents Actions. 1991b;33:104–107. - PubMed
-
- Alakarppa K, Tupala E, Mantere T, Sarkioja T, Rasanen P, Tarhanen J, et al. Effect of alcohol abuse on human brain histamine and tele-methylhistamine. Inflamm Res. 2002;51:40–41. - PubMed
-
- Alakarppa K, Tupala E, Mantere T, Sarkioja T, Rasanen P, Tarhanen J, et al. Alcoholics show altered histaminergic neurotransmission in several cortical areas–preliminary report. Inflamm Res. 2003;52:37–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

