Induction of pluripotency in bone marrow mononuclear cells via polyketal nanoparticle-mediated delivery of mature microRNAs
- PMID: 23489923
- PMCID: PMC3740735
- DOI: 10.1016/j.biomaterials.2013.02.005
Induction of pluripotency in bone marrow mononuclear cells via polyketal nanoparticle-mediated delivery of mature microRNAs
Abstract
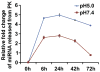
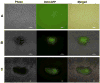
Since the successful generation of induced pluripotent stem cells (iPSC) from adult somatic cells using integrating-viral methods, various methods have been tried for iPSC generation using non-viral and non-integrating technique for clinical applications. Recently, various non-viral approaches such as protein, mRNA, microRNA, and small molecule transduction were developed to avoid genomic integration and generate stem cell-like cells from mouse and human fibroblasts. Despite these successes, there has been no successful generation of iPSC from bone marrow (BM)-derived hematopoietic cells derived using non-viral methods to date. Previous reports demonstrate the ability of polymeric micro and nanoparticles made from polyketals to deliver various molecules to macrophages. MicroRNA-loaded nanoparticles were created using the polyketal polymer PK3 (PK3-miR) and delivered to somatic cells for 6 days, resulting in the formation of colonies. Isolated cells from these colonies were assayed and substantial induction of the pluripotency markers Oct4, Sox2, and Nanog were detected. Moreover, colonies transferred to feeder layers also stained positive for pluripotency markers including SSEA-1. Here, we demonstrate successful activation of pluripotency-associated genes in mouse BM-mononuclear cells using embryonic stem cell (ESC)-specific microRNAs encapsulated in the acid sensitive polyketal PK3. These reprogramming results demonstrate that a polyketal-microRNA delivery vehicle can be used to generate various reprogrammed cells without permanent genetic manipulation in an efficient manner.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures




References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. - PubMed
-
- Huangfu D, Osafune K, Maehr R, Guo W, Eijkelenboom A, Chen S, et al. Induction of pluripotent stem cells from primary human fibroblasts with only Oct4 and Sox2. Nat Biotechnol. 2008;26:1269–75. - PubMed
-
- Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5:237–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

