Circumferential and functional re-entry of in vivo slow-wave activity in the porcine small intestine
- PMID: 23489929
- PMCID: PMC3781238
- DOI: 10.1111/nmo.12085
Circumferential and functional re-entry of in vivo slow-wave activity in the porcine small intestine
Abstract
Background: Slow-waves modulate the pattern of small intestine contractions. However, the large-scale spatial organization of intestinal slow-wave pacesetting remains uncertain because most previous studies have had limited resolution. This study applied high-resolution (HR) mapping to evaluate intestinal pacesetting mechanisms and propagation patterns in vivo.
Methods: HR serosal mapping was performed in anesthetized pigs using flexible arrays (256 electrodes; 32 × 8; 4 mm spacing), applied along the jejunum. Slow-wave propagation patterns, frequencies, and velocities were calculated. Slow-wave initiation sources were identified and analyzed by animation and isochronal activation mapping.
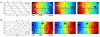
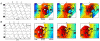
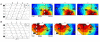
Key results: Analysis comprised 32 recordings from nine pigs (mean duration 5.1 ± 3.9 min). Slow-wave propagation was analyzed, and a total of 26 sources of slow-wave initiation were observed and classified as focal pacemakers (31%), sites of functional re-entry (23%) and circumferential re-entry (35%), or indeterminate sources (11%). The mean frequencies of circumferential and functional re-entry were similar (17.0 ± 0.3 vs 17.2 ± 0.4 cycle min(-1) ; P = 0.5), and greater than that of focal pacemakers (12.7 ± 0.8 cycle min(-1) ; P < 0.001). Velocity was anisotropic (12.9 ± 0.7 mm s(-1) circumferential vs 9.0 ± 0.7 mm s(-1) longitudinal; P < 0.05), contributing to the onset and maintenance of re-entry.
Conclusions & inferences: This study has shown multiple patterns of slow-wave initiation in the jejunum of anesthetized pigs. These results constitute the first description and analysis of circumferential re-entry in the gastrointestinal tract and functional re-entry in the in vivo small intestine. Re-entry can control the direction, pattern, and frequency of slow-wave propagation, and its occurrence and functional significance merit further investigation.
© 2013 Blackwell Publishing Ltd.
Figures



References
-
- Huizinga JD, Lammers WJEP. Gut peristalsis is coordinated by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:1–8. - PubMed
-
- Lammers WJEP, Ver Donck L, Stephen B, Smets D, Schuurkes JAJ. Origin and propagation of the slow wave in the canine stomach: the outlines of a gastric conduction system. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1200–10. - PubMed
-
- Hasler WL. Small intestinal motility. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Elsevier Academic Press; USA: 2006. pp. 935–64.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

