Functions of the centromere and kinetochore in chromosome segregation
- PMID: 23490282
- PMCID: PMC3687001
- DOI: 10.1016/j.ceb.2013.02.001
Functions of the centromere and kinetochore in chromosome segregation
Abstract
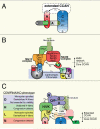
Centromeres play essential roles in equal chromosome segregation by directing the assembly of the microtubule binding kinetochore and serving as the cohesion site between sister chromatids. Here, we review the significant recent progress in our understanding of centromere protein assembly and how centromere proteins form the foundation of the kinetochore.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

