X-linked inhibitor of apoptosis regulates lung fibroblast resistance to Fas-mediated apoptosis
- PMID: 23492187
- PMCID: PMC3727886
- DOI: 10.1165/rcmb.2012-0224OC
X-linked inhibitor of apoptosis regulates lung fibroblast resistance to Fas-mediated apoptosis
Abstract
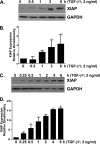
The accumulation of apoptosis-resistant fibroblasts within fibroblastic foci is a characteristic feature of idiopathic pulmonary fibrosis (IPF), but the mechanisms underlying apoptosis resistance remain unclear. A role for the inhibitor of apoptosis (IAP) protein family member X-linked inhibitor of apoptosis (XIAP) has been suggested by prior studies showing that (1) XIAP is localized to fibroblastic foci in IPF tissue and (2) prostaglandin E₂ suppresses XIAP expression while increasing fibroblast susceptibility to apoptosis. Based on these observations, we hypothesized that XIAP would be regulated by the profibrotic mediators transforming growth factor (TGF)β-1 and endothelin (ET)-1 and that increased XIAP would contribute to apoptosis resistance in IPF fibroblasts. To address these hypotheses, we examined XIAP expression in normal and IPF fibroblasts at baseline and in normal fibroblasts after treatment with TGF-β1 or ET-1. The role of XIAP in the regulation of fibroblast susceptibility to Fas-mediated apoptosis was examined using functional XIAP antagonists and siRNA silencing. In concordance with prior reports, fibroblasts from IPF lung tissue had increased resistance to apoptosis compared with normal lung fibroblasts. Compared with normal fibroblasts, IPF fibroblasts had significantly but heterogeneously increased basal XIAP expression. Additionally, TGF-β1 and ET-1 induced XIAP protein expression in normal fibroblasts. Inhibition or silencing of XIAP enhanced the sensitivity of lung fibroblasts to Fas-mediated apoptosis without causing apoptosis in the absence of Fas activation. Collectively, these findings support a mechanistic role for XIAP in the apoptosis-resistant phenotype of IPF fibroblasts.
Figures








References
-
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

