Differential regulation of pulmonary vascular cell growth by hypoxia-inducible transcription factor-1α and hypoxia-inducible transcription factor-2α
- PMID: 23492195
- PMCID: PMC3727885
- DOI: 10.1165/rcmb.2012-0107OC
Differential regulation of pulmonary vascular cell growth by hypoxia-inducible transcription factor-1α and hypoxia-inducible transcription factor-2α
Abstract
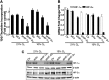
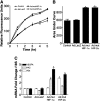
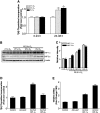
Hypoxia-inducible transcription factors HIF-1α and HIF-2α can contribute to pulmonary hypertension and vascular remodeling, but their mechanisms remain unknown. This study investigated the role of HIF-1α and HIF-2α in pulmonary artery endothelial and smooth muscle cells. The exposure of human pulmonary artery endothelial cells (HPAECs) to hypoxia (10% O₂ or 5% O₂) increased proliferation over 48 hours, compared with cells during normoxia (21% O₂). The adenovirus-mediated overexpression of HIF-2α that is transcriptionally active during normoxia (mutHIF-2α) increased HPAEC proliferation, whereas the overexpression of HIF-1α, which is transcriptionally active during normoxia (mutHIF-1α), exerted no effect. The knockdown of HIF-2α decreased proliferation during both hypoxia and normoxia. Both HIFs increased migration toward fibrinogen, used as a chemoattractant. In an angiogenesis tube formation assay, mutHIF-2α-transduced cells demonstrated increased tube formation, compared with the mutHIF-1α-transduced cells. In addition, the tubes formed in HIF-2α-transduced cells were more enduring than those in the other groups. In human pulmonary artery smooth muscle cells (HPASMCs), chronic exposure to hypoxia increased proliferation, compared with cells during normoxia. For HPASMCs transduced with adenoviral HIFs, HIF-1α increased proliferation, whereas HIF-2α exerted no such effect. Thus, HIF-1α and HIF-2α exert differential effects in isolated cells of the human pulmonary vasculature. This study demonstrates that HIF-2α plays a predominant role in the endothelial growth pertinent to the remodeling process. In contrast, HIF-1α appears to play a major role in pulmonary smooth muscle growth. The selective targeting of each HIF in specific target cells may more effectively counteract hypoxic pulmonary hypertension and vascular remodeling.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

