EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury
- PMID: 23492730
- PMCID: PMC3609137
- DOI: 10.1681/ASN.2012080871
EphrinB2 reverse signaling protects against capillary rarefaction and fibrosis after kidney injury
Abstract
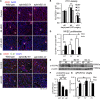
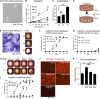
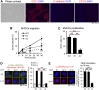
Microvascular disease, a characteristic of acute and chronic kidney diseases, leads to rarefaction of peritubular capillaries (PTCs), promoting secondary ischemic injury, which may be central to disease progression. Bidirectional signaling by EphB4 receptor and ephrinB2 ligand is critical for angiogenesis during murine development, suggesting that ephrinB2 reverse signaling may have a role in renal angiogenesis induced by injury or fibrosis. Here, we found that ephrinB2 reverse signaling is activated in the kidney only after injury. In mice lacking the PDZ intracellular signaling domain of ephrinB2 (ephrinB2 ΔV), angiogenesis was impaired and kidney injury led to increased PTC rarefaction and fibrosis. EphrinB2 ΔV primary kidney pericytes migrated more than wild-type pericytes and were less able to stabilize capillary tubes in three-dimensional culture and less able to stimulate synthesis of capillary basement membrane. EphrinB2 ΔV primary kidney microvascular endothelial cells migrated and proliferated less than wild-type microvascular endothelial cells in response to vascular endothelial growth factor A and showed less internalization and activation of vascular endothelial growth factor receptor-2. Taken together, these results suggest that PDZ domain-dependent ephrinB2 reverse signaling protects against PTC rarefaction by regulating angiogenesis and vascular stability during kidney injury. Furthermore, this signaling in kidney pericytes protects against pericyte-to-myofibroblast transition and myofibroblast activation, thereby limiting fibrogenesis.
Figures


Comment in
-
Getting the "inside" scoop on ephrinB2 signaling in pericytes and the effect on peritubular capillary stability.J Am Soc Nephrol. 2013 Mar;24(4):521-3. doi: 10.1681/ASN.2013020153. Epub 2013 Mar 14. J Am Soc Nephrol. 2013. PMID: 23492728 No abstract available.
References
-
- Basile DP: Rarefaction of peritubular capillaries following ischemic acute renal failure: A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 13: 1–7, 2004 - PubMed
-
- Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P: The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008 - PubMed
-
- Lin SL, Chang FC, Schrimpf C, Chen YT, Wu CF, Wu VC, Chiang WC, Kuhnert F, Kuo CJ, Chen YM, Wu KD, Tsai TJ, Duffield JS: Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178: 911–923, 2011 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

