Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis
- PMID: 23492732
- PMCID: PMC3636793
- DOI: 10.1681/ASN.2012070700
Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis
Abstract
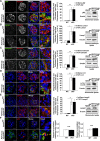
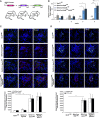
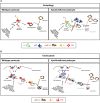
The molecular mechanisms that maintain podocytes and consequently, the integrity of the glomerular filtration barrier are incompletely understood. Here, we show that the class III phosphoinositide 3-kinase vacuolar protein sorting 34 (Vps34) plays a central role in modulating endocytic pathways, maintaining podocyte homeostasis. In mice, podocyte-specific conditional knockout of Vps34 led to early proteinuria, glomerular scarring, and death within 3-9 weeks of age. Vps34-deficient podocytes exhibited substantial vacuolization and foot process effacement. Although the formation of autophagosomes and autophagic flux were impaired, comparisons between podocyte-specific Vps34-deficient mice, autophagy-deficient mice, and doubly deficient mice suggested that defective autophagy was not primarily responsible for the severe phenotype caused by the loss of Vps34. In fact, Rab5-positive endosomal compartments, endocytosis, and fluid-phase uptake were severely disrupted in Vps34-deficient podocytes. Vps34 deficiency in nephrocytes, the podocyte-like cells of Drosophila melanogaster, resulted in a block between Rab5- and Rab7-positive endosomal compartments. In summary, these data identify Vps34 as a major regulator of endolysosomal pathways in podocytes and underline the fundamental roles of endocytosis and fluid-phase uptake for the maintenance of the glomerular filtration barrier.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

