Redox homeostasis: the linchpin in stem cell self-renewal and differentiation
- PMID: 23492768
- PMCID: PMC3613828
- DOI: 10.1038/cddis.2013.50
Redox homeostasis: the linchpin in stem cell self-renewal and differentiation
Abstract
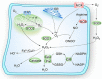
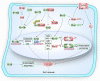
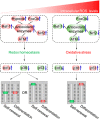
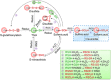
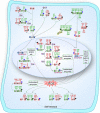
Stem cells are characterized by their unique ability of self-renewal to maintain the so-called stem cell pool. Over the past decades, reactive oxygen species (ROS) have been recognized as toxic aerobic metabolism byproducts that are harmful to stem cells, leading to DNA damage, senescence or cell death. Recently, a growing body of literature has shown that stem cells reside in redox niches with low ROS levels. The balance of Redox homeostasis facilitates stem cell self-renewal by an intricate network. Thus, to fully decipher the underlying molecular mechanisms involved in the maintenance of stem cell self-renewal, it is critical to address the important role of redox homeostasis in the regulation of self-renewal and differentiation of stem cells. In this regard, we will discuss the regulatory mechanisms involved in the subtly orchestrated balance of redox status in stem cells by scavenger antioxidant enzyme systems that are well monitored by the hypoxia niches and crucial redox regulators including forkhead homeobox type O family (FoxOs), apurinic/apyrimidinic (AP) endonuclease1/redox factor-1 (APE1/Ref-1), nuclear factor erythroid-2-related factor 2 (Nrf2) and ataxia telangiectasia mutated (ATM). We will also introduce several pivotal ROS-sensitive molecules, such as hypoxia-inducible factors, p38 mitogen-activated protein kinase (p38) and p53, involved in the redox-regulated stem cell self-renewal. Specifically, all the aforementioned molecules can act as 'redox sensors' by virtue of redox modifications of their cysteine residues, which are critically important in the control of protein function. Given the importance of redox homeostasis in the regulation of stem cell self-renewal, understanding the underlying molecular mechanisms involved will provide important new insights into stem cell biology.
Figures
References
-
- He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. - PubMed
-
- Simons BD, Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. - PubMed
-
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. - PubMed
-
- Ogasawara MA, Zhang H. Redox regulation and its emerging roles in stem cells and stem-like cancer cells. Antioxid Redox Signal. 2009;11:1107–1122. - PubMed
-
- D'Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

