Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson's disease
- PMID: 23492776
- PMCID: PMC3615743
- DOI: 10.1038/cddis.2013.73
Influence of microRNA deregulation on chaperone-mediated autophagy and α-synuclein pathology in Parkinson's disease
Abstract
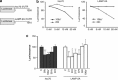
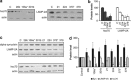
The presence of α-synuclein aggregates in the characteristic Lewy body pathology seen in idiopathic Parkinson's disease (PD), together with α-synuclein gene mutations in familial PD, places α-synuclein at the center of PD pathogenesis. Decreased levels of the chaperone-mediated autophagy (CMA) proteins LAMP-2A and hsc70 in PD brain samples suggests compromised α-synuclein degradation by CMA may underpin the Lewy body pathology. Decreased CMA protein levels were not secondary to the various pathological changes associated with PD, including mitochondrial respiratory chain dysfunction, increased oxidative stress and proteasomal inhibition. However, decreased hsc70 and LAMP-2A protein levels in PD brains were associated with decreases in their respective mRNA levels. MicroRNA (miRNA) deregulation has been reported in PD brains and we have identified eight miRNAs predicted to regulate LAMP-2A or hsc70 expression that were reported to be increased in PD. Using a luciferase reporter assay in SH-SY5Y cells, four and three of these miRNAs significantly decreased luciferase activity expressed upstream of the lamp-2a and hsc70 3'UTR sequences respectively. We confirmed that transfection of these miRNAs also decreased endogenous LAMP-2A and hsc70 protein levels respectively and resulted in significant α-synuclein accumulation. The analysis of PD brains confirmed that six and two of these miRNAs were significantly increased in substantia nigra compacta and amygdala respectively. These data support the hypothesis that decreased CMA caused by miRNA-induced downregulation of CMA proteins plays an important role in the α-synuclein pathology associated with PD, and opens up a new avenue to investigate PD pathogenesis.
Figures


Similar articles
-
Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson's disease.Mov Disord. 2015 Oct;30(12):1639-47. doi: 10.1002/mds.26141. Epub 2015 Jan 16. Mov Disord. 2015. PMID: 25594542
-
Chaperone-mediated autophagy markers in Parkinson disease brains.Arch Neurol. 2010 Dec;67(12):1464-72. doi: 10.1001/archneurol.2010.198. Epub 2010 Aug 9. Arch Neurol. 2010. PMID: 20697033
-
USP10 inhibits the degradation of α-synuclein, a pathogenic factor associated with Parkinson's disease, by inhibiting chaperone-mediated autophagy.J Biol Chem. 2025 Jul;301(7):110292. doi: 10.1016/j.jbc.2025.110292. Epub 2025 May 24. J Biol Chem. 2025. PMID: 40419127 Free PMC article.
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
-
[Alpha-synuclein interacted proteins: the relevance with the pathogenesis of Parkinson's disease].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2008 Sep;37(5):524-30. doi: 10.3785/j.issn.1008-9292.2008.05.019. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2008. PMID: 18925724 Review. Chinese.
Cited by
-
MicroRNAs in Parkinson's disease and emerging therapeutic targets.Neural Regen Res. 2017 Dec;12(12):1945-1959. doi: 10.4103/1673-5374.221147. Neural Regen Res. 2017. PMID: 29323027 Free PMC article. Review.
-
Therapeutic potential of autophagy-enhancing agents in Parkinson's disease.Mol Neurodegener. 2017 Jan 25;12(1):11. doi: 10.1186/s13024-017-0154-3. Mol Neurodegener. 2017. PMID: 28122627 Free PMC article. Review.
-
Impact of Chaperone-Mediated Autophagy in Brain Aging: Neurodegenerative Diseases and Glioblastoma.Front Aging Neurosci. 2021 Jan 28;12:630743. doi: 10.3389/fnagi.2020.630743. eCollection 2020. Front Aging Neurosci. 2021. PMID: 33633561 Free PMC article.
-
Autophagy in α-Synucleinopathies-An Overstrained System.Cells. 2021 Nov 12;10(11):3143. doi: 10.3390/cells10113143. Cells. 2021. PMID: 34831366 Free PMC article. Review.
-
Chaperone Mediated Autophagy in the Crosstalk of Neurodegenerative Diseases and Metabolic Disorders.Front Endocrinol (Lausanne). 2019 Jan 31;9:778. doi: 10.3389/fendo.2018.00778. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30766511 Free PMC article. Review.
References
-
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. - PubMed
-
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. Alpha-synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. - PubMed
-
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

