Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum
- PMID: 23492777
- PMCID: PMC3615735
- DOI: 10.1038/cddis.2013.74
Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum
Abstract
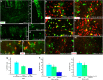
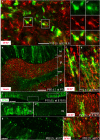
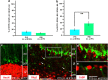
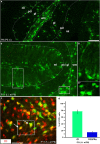
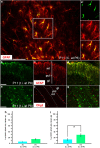
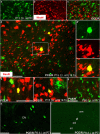
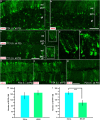
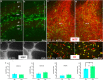
NG2 (nerve/glial antigen2)-expressing cells represent the largest population of postnatal progenitors in the central nervous system and have been classified as oligodendroglial progenitor cells, but the fate and function of these cells remain incompletely characterized. Previous studies have focused on characterizing these progenitors in the postnatal and adult subventricular zone and on analyzing the cellular and physiological properties of these cells in white and gray matter regions in the forebrain. In the present study, we examine the types of neural progeny generated by NG2 progenitors in the cerebellum by employing genetic fate mapping techniques using inducible Cre-Lox systems in vivo with two different mouse lines, the Plp-Cre-ER(T2)/Rosa26-EYFP and Olig2-Cre-ER(T2)/Rosa26-EYFP double-transgenic mice. Our data indicate that Olig2/Plp-positive NG2 cells display multipotential properties, primarily give rise to oligodendroglia but, surprisingly, also generate Bergmann glia, which are specialized glial cells in the cerebellum. The NG2+ cells also give rise to astrocytes, but not neurons. In addition, we show that glutamate signaling is involved in distinct NG2+ cell-fate/differentiation pathways and plays a role in the normal development of Bergmann glia. We also show an increase of cerebellar oligodendroglial lineage cells in response to hypoxic-ischemic injury, but the ability of NG2+ cells to give rise to Bergmann glia and astrocytes remains unchanged. Overall, our study reveals a novel Bergmann glia fate of Olig2/Plp-positive NG2 progenitors, demonstrates the differentiation of these progenitors into various functional glial cell types, and provides significant insights into the fate and function of Olig2/Plp-positive progenitor cells in health and disease.
Figures
Similar articles
-
Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo.J Neurosci. 2009 Jun 3;29(22):7256-70. doi: 10.1523/JNEUROSCI.5653-08.2009. J Neurosci. 2009. PMID: 19494148 Free PMC article.
-
Early postnatal GFAP-expressing cells produce multilineage progeny in cerebrum and astrocytes in cerebellum of adult mice.Brain Res. 2013 Sep 26;1532:14-20. doi: 10.1016/j.brainres.2013.08.003. Epub 2013 Aug 9. Brain Res. 2013. PMID: 23939222
-
Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex.J Neurosci. 2008 Oct 8;28(41):10434-42. doi: 10.1523/JNEUROSCI.2831-08.2008. J Neurosci. 2008. PMID: 18842903 Free PMC article.
-
Astrocytes and NG2-glia: what's in a name?J Anat. 2005 Dec;207(6):687-93. doi: 10.1111/j.1469-7580.2005.00489.x. J Anat. 2005. PMID: 16367796 Free PMC article. Review.
-
Morphological and physiological interactions of NG2-glia with astrocytes and neurons.J Anat. 2007 Jun;210(6):661-70. doi: 10.1111/j.1469-7580.2007.00729.x. Epub 2007 Apr 25. J Anat. 2007. PMID: 17459143 Free PMC article. Review.
Cited by
-
Defects in translation-dependent quality control pathways lead to convergent molecular and neurodevelopmental pathology.Elife. 2021 Apr 26;10:e66904. doi: 10.7554/eLife.66904. Elife. 2021. PMID: 33899734 Free PMC article.
-
Oligodendroglial defects during quakingviable cerebellar development.Dev Neurobiol. 2016 Sep;76(9):972-82. doi: 10.1002/dneu.22369. Epub 2015 Dec 22. Dev Neurobiol. 2016. PMID: 26645409 Free PMC article.
-
Four main therapeutic keys for Parkinson's disease: A mini review.Iran J Basic Med Sci. 2019 Jul;22(7):716-721. doi: 10.22038/ijbms.2019.33659.8025. Iran J Basic Med Sci. 2019. PMID: 32373291 Free PMC article. Review.
-
FBXW7 regulates MYRF levels to control myelin capacity and homeostasis in the adult central nervous system.Nat Commun. 2025 Aug 21;16(1):7822. doi: 10.1038/s41467-025-62715-9. Nat Commun. 2025. PMID: 40841354
-
Organization of the ventricular zone of the cerebellum.Front Cell Neurosci. 2022 Jul 25;16:955550. doi: 10.3389/fncel.2022.955550. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35959470 Free PMC article. Review.
References
-
- Levine JM, Reynolds R, Fawcett JW. The oligodendrocyte precursor cell in health and disease. Trends Neurosci. 2001;24:39–47. - PubMed
-
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J Neurocytol. 2002;31:423–435. - PubMed
-
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. - PubMed
-
- Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289:1754–1757. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

