Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics
- PMID: 23492811
- PMCID: PMC3663562
- DOI: 10.1093/toxsci/kft071
Ozone-induced injury and oxidative stress in bronchiolar epithelium are associated with altered pulmonary mechanics
Abstract
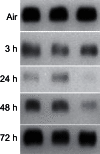
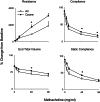
In these studies, we analyzed the effects of ozone on bronchiolar epithelium. Exposure of rats to ozone (2 ppm, 3 h) resulted in rapid (within 3 h) and persistent (up to 72 h) histological changes in the bronchiolar epithelium, including hypercellularity, loss of cilia, and necrotizing bronchiolitis. Perivascular edema and vascular congestion were also evident, along with a decrease in Clara cell secretory protein in bronchoalveolar lavage, which was maximal 24 h post-exposure. Ozone also induced the appearance of 8-hydroxy-2'-deoxyguanosine, Ym1, and heme oxygenase-1 in the bronchiolar epithelium. This was associated with increased expression of cleaved caspase-9 and beclin-1, indicating initiation of apoptosis and autophagy. A rapid and persistent increase in galectin-3, a regulator of epithelial cell apoptosis, was also observed. Following ozone exposure (3-24 h), increased expression of cyclooxygenase-2, inducible nitric oxide synthase, and arginase-1 was noted in bronchiolar epithelium. Ozone-induced injury and oxidative stress in bronchiolar epithelium were linked to methacholine-induced alterations in pulmonary mechanics. Thus, significant increases in lung resistance and elastance, along with decreases in lung compliance and end tidal volume, were observed at higher doses of methacholine. This indicates that ozone causes an increase in effective stiffness of the lung as a consequence of changes in the conducting airways. Collectively, these studies demonstrate that bronchiolar epithelium is highly susceptible to injury and oxidative stress induced by acute exposure to ozone; moreover, this is accompanied by altered lung functioning.
Keywords: bronchiole; epithelium; oxidative stress; ozone; pulmonary mechanics.
Figures





References
-
- Aoshiba K., Koinuma M., Yokohori N., Nagai A. (2003). Immunohistochemical evaluation of oxidative stress in murine lungs after cigarette smoke exposure. Inhal. Toxicol. 15, 1029–1038 - PubMed
-
- Bates J. H., Lauzon A. M. (2007). Parenchymal tethering, airway wall stiffness, and the dynamics of bronchoconstriction. J. Appl. Physiol. 102, 1912–1920 - PubMed
-
- Bates J. H., Lutchen K. R. (2005). The interface between measurement and modeling of peripheral lung mechanics. Respir. Physiol. Neurobiol. 148, 153–164 - PubMed
-
- Broeckaert F., Clippe A., Wattiez R., Falmagne P., Bernard A. (2003). Lung hyperpermeability, Clara-cell secretory protein (CC16), and susceptibility to ozone of five inbred strains of mice. Inhal. Toxicol. 15, 1209–1230 - PubMed
-
- Cai Y., Kumar R. K., Zhou J., Foster P. S., Webb D. C. (2009). Ym1/2 promotes Th2 cytokine expression by inhibiting 12/15(S)-lipoxygenase: Identification of a novel pathway for regulating allergic inflammation. J. Immunol. 182, 5393–5399 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

