Breathing-in epigenetic change with vitamin C
- PMID: 23492828
- PMCID: PMC3615655
- DOI: 10.1038/embor.2013.29
Breathing-in epigenetic change with vitamin C
Abstract
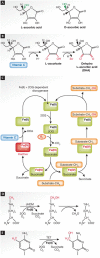
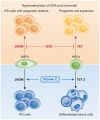
Vitamin C is an antioxidant that maintains the activity of iron and α-ketoglutarate-dependent dioxygenases. Despite these enzymes being implicated in a wide range of biological pathways, vitamin C is rarely included in common cell culture media. Recent studies show that reprogramming of pluripotent stem cells is enhanced when vitamin C is present, thereby illustrating previous limitations in reprogramming cultures. Here, we summarize understanding of dioxygenase function in reprogramming and epigenetic regulation. The available data suggest a link between dioxygenase function and stem cell differentiation, which is exposed to environmental influence and is relevant for human disease.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Peterkofsky B (1991) Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr 54: 1135S–1140S - PubMed
-
- Baron JH (2009) Sailors' scurvy before and after James Lind—a reassessment. Nutr Rev 67: 315–332 - PubMed
-
- Nelson PJ, Pruitt RE, Henderson LL, Jenness R, Henderson LM (1981) Effect of ascorbic acid deficiency on the in vivo synthesis of carnitine. Biochim Biophys Acta 672: 123–127 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

