Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4
- PMID: 23493551
- PMCID: PMC3612666
- DOI: 10.1073/pnas.1303094110
Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4
Abstract
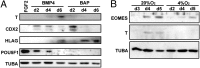
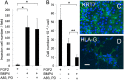
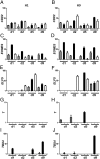
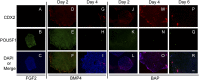
Human ES cells (hESC) exposed to bone morphogenic protein 4 (BMP4) in the absence of FGF2 have become widely used for studying trophoblast development, but the soundness of this model has been challenged by others, who concluded that differentiation was primarily toward mesoderm rather than trophoblast. Here we confirm that hESC grown under the standard conditions on a medium conditioned by mouse embryonic fibroblasts in the presence of BMP4 and absence of FGF2 on a Matrigel substratum rapidly convert to an epithelium that is largely KRT7(+) within 48 h, with minimal expression of mesoderm markers, including T (Brachyury). Instead, they begin to express a series of trophoblast markers, including HLA-G, demonstrate invasive properties that are independent of the continued presence of BMP4 in the medium, and, over time, produce extensive amounts of human chorionic gonadotropin, progesterone, placental growth factor, and placental lactogen. This process of differentiation is not dependent on conditioning of the medium by mouse embryonic fibroblasts and is accelerated in the presence of inhibitors of Activin and FGF2 signaling, which at day 2 provide colonies that are entirely KRT7(+) and in which the majority of cells are transiently CDX2(+). Colonies grown on two chemically defined media, including the one in which BMP4 was reported to drive mesoderm formation, also differentiate at least partially to trophoblast in response to BMP4. The experiments demonstrate that the in vitro BMP4/hESC model is valid for studying the emergence and differentiation of trophoblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Xu RH, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20(12):1261–1264. - PubMed
-
- Golos TG, Pollastrini LM, Gerami-Naini B. Human embryonic stem cells as a model for trophoblast differentiation. Semin Reprod Med. 2006;24(5):314–321. - PubMed
-
- Zhang P, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111(4):1933–1941. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

