X-box binding protein 1 is essential for insulin regulation of pancreatic α-cell function
- PMID: 23493568
- PMCID: PMC3712068
- DOI: 10.2337/db12-1747
X-box binding protein 1 is essential for insulin regulation of pancreatic α-cell function
Abstract
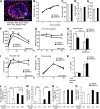
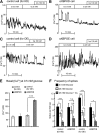
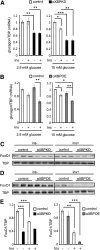
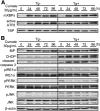
Patients with type 2 diabetes (T2D) often exhibit hyperglucagonemia despite hyperglycemia, implicating defective α-cell function. Although endoplasmic reticulum (ER) stress has been suggested to underlie β-cell dysfunction in T2D, its role in α-cell biology remains unclear. X-box binding protein 1 (XBP1) is a transcription factor that plays a crucial role in the unfolded protein response (UPR), and its deficiency in β-cells has been reported to impair insulin secretion, leading to glucose intolerance. To evaluate the role of XBP1 in α-cells, we created complementary in vivo (α-cell-specific XBP1 knockout [αXBPKO] mice) and in vitro (stable XBP1 knockdown α-cell line [αXBPKD]) models. The αXBPKO mice exhibited glucose intolerance, mild insulin resistance, and an inability to suppress glucagon secretion after glucose stimulation. αXBPKD cells exhibited activation of inositol-requiring enzyme 1, an upstream activator of XBP1, leading to phosphorylation of Jun NH2-terminal kinase. Interestingly, insulin treatment of αXBPKD cells reduced tyrosine phosphorylation of insulin receptor substrate 1 (IRS1) (pY(896)) and phosphorylation of Akt while enhancing serine phosphorylation (pS(307)) of IRS1. Consequently, the αXBPKD cells exhibited blunted suppression of glucagon secretion after insulin treatment in the presence of high glucose. Together, these data indicate that XBP1 deficiency in pancreatic α-cells induces altered insulin signaling and dysfunctional glucagon secretion.
Figures




References
-
- Reaven GM, Chen YD, Golay A, Swislocki AL, Jaspan JB. Documentation of hyperglucagonemia throughout the day in nonobese and obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1987;64:106–110 - PubMed
-
- Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 1987;36:274–283 - PubMed
-
- Ravier MA, Rutter GA. Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic alpha-cells. Diabetes 2005;54:1789–1797 - PubMed
-
- Gerich JE, Tsalikian E, Lorenzi M, et al. Normalization of fasting hyperglucagonemia and excessive glucagon responses to intravenous arginine in human diabetes mellitus by prolonged infusion of insulin. J Clin Endocrinol Metab 1975;41:1178–1180 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

