Mining the "glycocode"--exploring the spatial distribution of glycans in gastrointestinal mucin using force spectroscopy
- PMID: 23493619
- PMCID: PMC3659345
- DOI: 10.1096/fj.12-221416
Mining the "glycocode"--exploring the spatial distribution of glycans in gastrointestinal mucin using force spectroscopy
Abstract
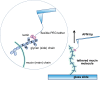
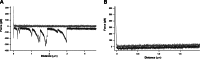
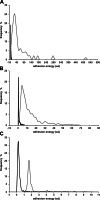
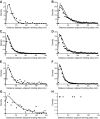
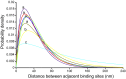
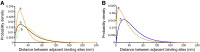
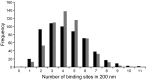
Mucins are the main components of the gastrointestinal mucus layer. Mucin glycosylation is critical to most intermolecular and intercellular interactions. However, due to the highly complex and heterogeneous mucin glycan structures, the encoded biological information remains largely encrypted. Here we have developed a methodology based on force spectroscopy to identify biologically accessible glycoepitopes in purified porcine gastric mucin (pPGM) and purified porcine jejunal mucin (pPJM). The binding specificity of lectins Ricinus communis agglutinin I (RCA), peanut (Arachis hypogaea) agglutinin (PNA), Maackia amurensis lectin II (MALII), and Ulex europaeus agglutinin I (UEA) was utilized in force spectroscopy measurements to quantify the affinity and spatial distribution of their cognate sugars at the molecular scale. Binding energy of 4, 1.6, and 26 aJ was determined on pPGM for RCA, PNA, and UEA. Binding was abolished by competition with free ligands, demonstrating the validity of the affinity data. The distributions of the nearest binding site separations estimated the number of binding sites in a 200-nm mucin segment to be 4 for RCA, PNA, and UEA, and 1.8 for MALII. Binding site separations were affected by partial defucosylation of pPGM. Furthermore, we showed that this new approach can resolve differences between gastric and jejunum mucins.
Keywords: adhesion; atomic force microscopy; lectin-carbohydrate interaction; molecular recognition.
Figures



References
-
- Johansson M. E., Ambort D., Pelaseyed T., Schütte A., Gustafsson J. K., Ermund A., Subramani. D. B., Holmén-Larsson. J. M., Thomsson. K. A., Bergström. J. H., van der Post. S., Rodriguez-Piñeiro A. M., Sjövall H., Bäckström M., Hansson G. C. (2011) Composition and functional role of the mucus layers in the intestine. Cell. Mol. Life Sci. 68, 3635–3641 - PMC - PubMed
-
- Godl K., Johansson M. E. V., Lidell M. E., Mörgelin M., Karlsson H., Olson F. J., Gum J. R., Jr., Kim Y. S., Hansson G. C. (2002) The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J. Biol. Chem. 277, 47248–47256 - PubMed
-
- Jonckheere N., Van Seuningen I. (2010) The membrane-bound mucins: from cell signalling to transcriptional regulation and expression in epithelial cancers. Biochimie (Paris) 92, 1–11 - PubMed
-
- Robbe-Masselot C., Maes E., Rousset M., Michalski J. C., Capon C. (2009) Glycosylation of human fetal mucins: a similar repertoire of O-glycans along the intestinal tract. Glycoconj. J. 26, 397–413 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

