Teaching basic principles of neuroscience with computer simulations
- PMID: 23493644
- PMCID: PMC3592631
Teaching basic principles of neuroscience with computer simulations
Abstract
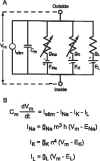
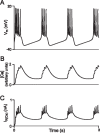
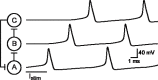
It is generally believed that students learn best through activities that require their direct participation. By using simulations as a tool for learning neuroscience, students are directly engaged in the activity and obtain immediate feedback and reinforcement. This paper describes a series of biophysical models and computer simulations that can be used by educators and students to explore a variety of basic principles in neuroscience. The paper also suggests 'virtual laboratory' exercises that students may conduct to further examine biophysical processes underlying neural function. First, the Hodgkin and Huxley (HH) model is presented. The HH model is used to illustrate the action potential, threshold phenomena, and nonlinear dynamical properties of neurons (e.g., oscillations, postinhibitory rebound excitation). Second, the Morris-Lecar (ML) model is presented. The ML model is used to develop a model of a bursting neuron and to illustrate modulation of neuronal activity by intracellular ions. Lastly, principles of synaptic transmission are presented in small neural networks, which illustrate oscillatory behavior, excitatory and inhibitory postsynaptic potentials, and temporal summation.
Keywords: Hodgkin-Huxley; SNNAP; graduate; modeling; neural networks; neurons; synapses; undergraduate.
Figures








References
-
- Abbott L, Marder E. Modeling small networks. In: Koch C, Segev I, editors. Methods in neuronal modeling: from ions to networks. 2nd edition. Cambridge, MA: The MIT Press; 1998. pp. 363–410.
-
- Abraham RH, Shaw CD. Dynamics: the geometry of behavior. 2nd edition. Redwood City, CA: Addison-Wesley; 1992.
-
- Av-Ron E, Parnas H, Segel LA. A minimal biophysical model for an excitable and oscillatory neuron. Biol Cybern. 1991;65:487–500. - PubMed
-
- Av-Ron E, Vidal PP. Intrinsic membrane properties and dynamics of medial vestibular neurons: a simulation. Biol Cybern. 1999;80:383–392. - PubMed
-
- Av-Ron E, Cai Y, Byrne JH, Baxter DA. Soc. Neurosci. Abstr. Washington, DC: Society for Neuroscience; 2004. An interactive and educational tutorial for version 8 of SNNAP (Simulator for Neural Networks and Action Potentials) Program No. 27.13, 2004 Abstract Viewer/Itinerary Planner. Online: sfn.scholarone.com/itin2004/
Grants and funding
LinkOut - more resources
Full Text Sources
