The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis
- PMID: 23493728
- PMCID: PMC3654748
- DOI: 10.1093/infdis/jit099
The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis
Abstract
Background: Correlates of immune protection in patients with human immunodeficiency virus (HIV)-associated cryptococcal meningitis are poorly defined. A clearer understanding of these immune responses is essential to inform rational development of immunotherapies.
Methods: Cryptococcal-specific peripheral CD4(+) T-cell responses were measured in 44 patients with HIV-associated cryptococcal meningitis at baseline and during follow-up. Responses were assessed following ex vivo cryptococcal mannoprotein stimulation, using 13-color flow-cytometry. The relationships between cryptococcal-specific CD4(+) T-cell responses, clinical parameters at presentation, and outcome were investigated.
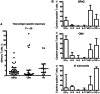
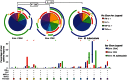
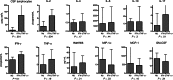
Results: Cryptococcal-specific CD4(+) T-cell responses were characterized by the production of macrophage inflammatory protein 1α, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α). Conversely, minimal interleukin 4 and interleukin 17 production was detected. Patients surviving to 2 weeks had significantly different functional CD4(+) T-cell responses as compared to those who died. Patients with a response predominantly consisting of IFN-γ or TNF-α production had a 2-week mortality of 0% (0/20), compared with 25% (6/24) in those without this response (P = .025). Such patients also had lower fungal burdens (10 400 vs 390 000 colony-forming units/mL; P < .001), higher cerebrospinal fluid lymphocyte counts (122 vs 8 cells/μL; P < .001), and a trend toward faster rates of clearance of infection.
Conclusions: The phenotype of the peripheral CD4(+) T-cell response to Cryptococcus was associated with disease severity and outcome in HIV-associated cryptococcal meningitis. IFN-γ/TNF-α-predominant responses were associated with survival.
Keywords: CMV; HIV; TB; cryptococcus neoformans; flow cytometry; memory T cells.
Figures



Comment in
-
Advancing translational immunology in HIV-associated cryptococcal meningitis.J Infect Dis. 2013 Jun 15;207(12):1793-5. doi: 10.1093/infdis/jit102. Epub 2013 Mar 14. J Infect Dis. 2013. PMID: 23493727 Free PMC article. No abstract available.
References
-
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. - PubMed
-
- Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Ready made’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi—the Cryptococcus neoformans paradigm. Curr Opin Microbiol. 2003;6:332–7. - PubMed
-
- Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. - PubMed
-
- Hill JO, Aguirre KM. CD4+ T cell-dependent acquired state of immunity that protects the brain against Cryptococcus neoformans. J Immunol. 1994;152:2344–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

