Generation of mice encoding a conditional allele of Nkx2.2
- PMID: 23494546
- PMCID: PMC3713092
- DOI: 10.1007/s11248-013-9700-0
Generation of mice encoding a conditional allele of Nkx2.2
Abstract
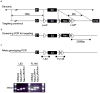
Nkx2.2 is a homeobox transcription factor that is expressed in the pancreas, intestine and central nervous system (CNS) during embryogenesis and in the adult. In mice, global deletion of Nkx2.2 results in cell mis-specification in each of the tissues where it is expressed, and the null mice die as neonates with severe hyperglycemia. Although a whole body knockout demonstrates the importance of Nkx2.2 in cell specification and postnatal viability, it precludes assessment of the cell-autonomous and postnatal functions of Nkx2.2. In this study we report the generation and functional characterization of mice encoding a conditional allele of Nkx2.2. We demonstrate the functional integrity of the conditional Nkx2.2 allele and report successful in vivo deletion using a pancreas-specific Cre recombinase. The pancreas-specific deletion of Nkx2.2 results in similar defects found in the Nkx2.2 null pancreas and the mice die shortly after birth, demonstrating that the neonatal lethality associated with the null allele is caused by pancreatic islet dysfunction. The generation of a conditional Nkx2.2 allele provides an important tool for identifying the cell-autonomous and postnatal activities of Nkx2.2 in establishing and maintaining cell type identities and functions in the pancreas, intestine and CNS.
Figures



References
-
- Banks AS, Kim-Muller JY, Mastracci TL, Kofler NM, Qiang L, Haeusler RA, Jurczak MJ, Laznik D, Heinrich G, Samuel VT, Shulman GI, Papaioannou VE, Accili D. Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylation-defective alleles in mice. Cell Metab. 2011;14 (5):587–597. - PMC - PubMed
-
- Briscoe J, Sussel L, Serup P, Hartigan-O’Connor D, Jessell TM, Rubenstein JL, Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999;398 (6728):622–627. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

