Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors
- PMID: 23495178
- PMCID: PMC4540234
- DOI: 10.1002/stem.1372
Developing rods transplanted into the degenerating retina of Crx-knockout mice exhibit neural activity similar to native photoreceptors
Abstract
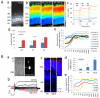
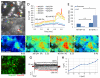
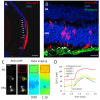
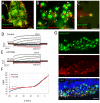
Replacement of dysfunctional or dying photoreceptors offers a promising approach for retinal neurodegenerative diseases, including age-related macular degeneration and retinitis pigmentosa. Several studies have demonstrated the integration and differentiation of developing rod photoreceptors when transplanted in wild-type or degenerating retina; however, the physiology and function of the donor cells are not adequately defined. Here, we describe the physiological properties of developing rod photoreceptors that are tagged with green fluorescent protein (GFP) driven by the promoter of rod differentiation factor, Nrl. GFP-tagged developing rods show Ca(2 +) responses and rectifier outward currents that are smaller than those observed in fully developed photoreceptors, suggesting their immature developmental state. These immature rods also exhibit hyperpolarization-activated current (Ih ) induced by the activation of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels. When transplanted into the subretinal space of wild-type or retinal degeneration mice, GFP-tagged developing rods can integrate into the photoreceptor outer nuclear layer in wild-type mouse retina and exhibit Ca(2 +) responses and membrane current comparable to native rod photoreceptors. A proportion of grafted rods develop rhodopsin-positive outer segment-like structures within 2 weeks after transplantation into the retina of Crx-knockout mice and produce rectifier outward current and Ih upon membrane depolarization and hyperpolarization. GFP-positive rods derived from induced pluripotent stem (iPS) cells also display similar membrane current Ih as native developing rod photoreceptors, express rod-specific phototransduction genes, and HCN-1 channels. We conclude that Nrl-promoter-driven GFP-tagged donor photoreceptors exhibit physiological characteristics of rods and that iPS cell-derived rods in vitro may provide a renewable source for cell-replacement therapy.
Copyright © 2013 AlphaMed Press.
Figures
Similar articles
-
Different effects of valproic acid on photoreceptor loss in Rd1 and Rd10 retinal degeneration mice.Mol Vis. 2014 Nov 4;20:1527-44. eCollection 2014. Mol Vis. 2014. PMID: 25489226 Free PMC article.
-
FIZ1 is expressed during photoreceptor maturation, and synergizes with NRL and CRX at rod-specific promoters in vitro.Exp Eye Res. 2007 Feb;84(2):349-60. doi: 10.1016/j.exer.2006.10.009. Epub 2006 Dec 4. Exp Eye Res. 2007. PMID: 17141759 Free PMC article.
-
RETINA-specific expression of Kcnv2 is controlled by cone-rod homeobox (Crx) and neural retina leucine zipper (Nrl).Adv Exp Med Biol. 2014;801:31-41. doi: 10.1007/978-1-4614-3209-8_5. Adv Exp Med Biol. 2014. PMID: 24664678
-
Regulation of photoreceptor gene expression by Crx-associated transcription factor network.Brain Res. 2008 Feb 4;1192:114-33. doi: 10.1016/j.brainres.2007.06.036. Epub 2007 Jun 30. Brain Res. 2008. PMID: 17662965 Free PMC article. Review.
-
Advances in repairing the degenerate retina by rod photoreceptor transplantation.Biotechnol Adv. 2014 Mar-Apr;32(2):485-91. doi: 10.1016/j.biotechadv.2014.01.001. Epub 2014 Jan 8. Biotechnol Adv. 2014. PMID: 24412415 Free PMC article. Review.
Cited by
-
Tissue engineering of the retina: from organoids to microfluidic chips.J Tissue Eng. 2021 Dec 10;12:20417314211059876. doi: 10.1177/20417314211059876. eCollection 2021 Jan-Dec. J Tissue Eng. 2021. PMID: 34917332 Free PMC article. Review.
-
Embryonic stem cell-derived photoreceptor precursor cells differentiated by coculture with RPE cells.Mol Vis. 2021 May 9;27:288-299. eCollection 2021. Mol Vis. 2021. PMID: 34012231 Free PMC article.
-
Photoreceptor Transplantation in Late Stage Retinal Degeneration.Invest Ophthalmol Vis Sci. 2016 Apr 1;57(5):ORSFg1-7. doi: 10.1167/iovs.15-17659. Invest Ophthalmol Vis Sci. 2016. PMID: 27116664 Free PMC article. Review.
-
Quantitative and Qualitative Evaluation of Photoreceptor Synapses in Developing, Degenerating and Regenerating Retinas.Front Cell Neurosci. 2019 Feb 11;13:16. doi: 10.3389/fncel.2019.00016. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30804754 Free PMC article.
-
Increasing cell culture density during a developmental window prevents fated rod precursors derailment toward hybrid rod-glia cells.Sci Rep. 2023 Apr 13;13(1):6025. doi: 10.1038/s41598-023-32571-y. Sci Rep. 2023. PMID: 37055439 Free PMC article.
References
-
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. - PubMed
-
- Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. - PubMed
-
- Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

