In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids
- PMID: 23495239
- PMCID: PMC3791157
- DOI: 10.1002/adhm.201300002
In vivo treatment of Propionibacterium acnes infection with liposomal lauric acids
Abstract
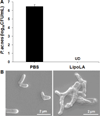
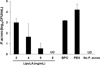
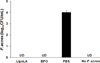
Propionibacterium acnes (P. acnes) is a Gram-positive bacterium strongly associated with acne infection. While many antimicrobial agents have been used in clinic to treat acne infection by targeting P. acnes, these existing anti-acne agents usually produce considerable side effects. Herein, the development and evaluation of liposomal lauric acids (LipoLA) is reported as a new, effective and safe therapeutic agent for the treatment of acne infection. By incorporating lauric acids into the lipid bilayer of liposomes, it is observed that the resulting LipoLA readily fuse with bacterial membranes, causing effective killing of P. acnes by disrupting bacterial membrane structures. Using a mouse ear model, we demonstrated that the bactericidal property of LipoLA against P. acne is well preserved at physiological conditions. Topically applying LipoLA in a gel form onto the infectious sites leads to eradication of P. acnes bacteria in vivo. Further skin toxicity studies show that LipoLA does not induce acute toxicity to normal mouse skin, while benzoyl peroxide and salicylic acid, the two most popular over-the-counter acne medications, generate moderate to severe skin irritation within 24 h. These results suggest that LipoLA hold a high therapeutic potential for the treatment of acne infection and other P. acnes related diseases.
Keywords: Propionibacterium acnes; antimicrobial delivery; bacterial infection; free fatty acid; nanoparticle.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



Similar articles
-
The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes.Biomaterials. 2009 Oct;30(30):6035-40. doi: 10.1016/j.biomaterials.2009.07.033. Epub 2009 Aug 8. Biomaterials. 2009. PMID: 19665786 Free PMC article.
-
Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris.J Invest Dermatol. 2009 Oct;129(10):2480-8. doi: 10.1038/jid.2009.93. Epub 2009 Apr 23. J Invest Dermatol. 2009. PMID: 19387482 Free PMC article.
-
Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: a comparative study with lauric acid.J Dermatol Sci. 2014 Mar;73(3):232-40. doi: 10.1016/j.jdermsci.2013.10.010. Epub 2013 Nov 7. J Dermatol Sci. 2014. PMID: 24284257
-
Propionibacterium acnes: infection beyond the skin.Expert Rev Anti Infect Ther. 2011 Dec;9(12):1149-56. doi: 10.1586/eri.11.137. Expert Rev Anti Infect Ther. 2011. PMID: 22114965 Review.
-
Acne and Propionibacterium acnes.Clin Dermatol. 2004 Sep-Oct;22(5):375-9. doi: 10.1016/j.clindermatol.2004.03.005. Clin Dermatol. 2004. PMID: 15556721 Review.
Cited by
-
Mechanism of antibacterial activity of liposomal linolenic acid against Helicobacter pylori.PLoS One. 2015 Mar 20;10(3):e0116519. doi: 10.1371/journal.pone.0116519. eCollection 2015. PLoS One. 2015. PMID: 25793403 Free PMC article.
-
Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications.Int J Mol Sci. 2018 Apr 8;19(4):1114. doi: 10.3390/ijms19041114. Int J Mol Sci. 2018. PMID: 29642500 Free PMC article. Review.
-
Intracellular Staphylococcus aureus Elicits the Production of Host Very Long-Chain Saturated Fatty Acids with Antimicrobial Activity.Metabolites. 2019 Jul 20;9(7):148. doi: 10.3390/metabo9070148. Metabolites. 2019. PMID: 31330837 Free PMC article.
-
Anti-Inflammatory and Antibacterial Activity Constituents from the Stem of Cinnamomum validinerve.Molecules. 2020 Jul 25;25(15):3382. doi: 10.3390/molecules25153382. Molecules. 2020. PMID: 32722482 Free PMC article.
-
Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy.Int J Nanomedicine. 2016 May 11;11:1987-2007. doi: 10.2147/IJN.S104701. eCollection 2016. Int J Nanomedicine. 2016. PMID: 27274231 Free PMC article. Review.
References
-
- Dreno B, Poli F. Dermatology. 2003;206:7–10. - PubMed
-
- Gollnick H. Drugs. 2003;63:1579–1596. - PubMed
-
- Toyoda M, Nakamura M, Morohashi M. Eur. J. Dermatol. 2002;12:422–427. - PubMed
-
- Webster GF. J. Am. Acad. Dermatol. 1995;33:247–253. - PubMed
-
- Garg VK, Sinha S, Sarkar R. Dermatol. Surg. 2009;35:59–65. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

