Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial
- PMID: 23496872
- PMCID: PMC3599745
- DOI: 10.1186/1741-7015-11-68
Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial
Abstract
Background: Early rapid fluid resuscitation (boluses) in African children with severe febrile illnesses increases the 48-hour mortality by 3.3% compared with controls (no bolus). We explored the effect of boluses on 48-hour all-cause mortality by clinical presentation at enrolment, hemodynamic changes over the first hour, and on different modes of death, according to terminal clinical events. We hypothesize that boluses may cause excess deaths from neurological or respiratory events relating to fluid overload.
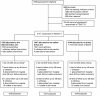
Methods: Pre-defined presentation syndromes (PS; severe acidosis or severe shock, respiratory, neurological) and predominant terminal clinical events (cardiovascular collapse, respiratory, neurological) were described by randomized arm (bolus versus control) in 3,141 severely ill febrile children with shock enrolled in the Fluid Expansion as Supportive Therapy (FEAST) trial. Landmark analyses were used to compare early mortality in treatment groups, conditional on changes in shock and hypoxia parameters. Competing risks methods were used to estimate cumulative incidence curves and sub-hazard ratios to compare treatment groups in terms of terminal clinical events.
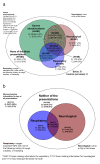
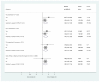
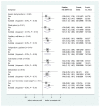
Results: Of 2,396 out of 3,141 (76%) classifiable participants, 1,647 (69%) had a severe metabolic acidosis or severe shock PS, 625 (26%) had a respiratory PS and 976 (41%) had a neurological PS, either alone or in combination. Mortality was greatest among children fulfilling criteria for all three PS (28% bolus, 21% control) and lowest for lone respiratory (2% bolus, 5% control) or neurological (3% bolus, 0% control) presentations. Excess mortality in bolus arms versus control was apparent for all three PS, including all their component features. By one hour, shock had resolved (responders) more frequently in bolus versus control groups (43% versus 32%, P <0.001), but excess mortality with boluses was evident in responders (relative risk 1.98, 95% confidence interval 0.94 to 4.17, P = 0.06) and 'non-responders' (relative risk 1.67, 95% confidence interval 1.23 to 2.28, P = 0.001), with no evidence of heterogeneity (P = 0.68). The major difference between bolus and control arms was the higher proportion of cardiogenic or shock terminal clinical events in bolus arms (n = 123; 4.6% versus 2.6%, P = 0.008) rather than respiratory (n = 61; 2.2% versus 1.3%, P = 0.09) or neurological (n = 63, 2.1% versus 1.8%, P = 0.6) terminal clinical events.
Conclusions: Excess mortality from boluses occurred in all subgroups of children. Contrary to expectation, cardiovascular collapse rather than fluid overload appeared to contribute most to excess deaths with rapid fluid resuscitation. These results should prompt a re-evaluation of evidence on fluid resuscitation for shock and a re-appraisal of the rate, composition and volume of resuscitation fluids.
Trial registration: ISRCTN69856593.
Figures



Comment in
-
Causes of death after fluid bolus resuscitation: new insights from FEAST.BMC Med. 2013 Mar 14;11:67. doi: 10.1186/1741-7015-11-67. BMC Med. 2013. PMID: 23497460 Free PMC article.
References
-
- Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, Akech SO, Nyeko R, Mtove G, Reyburn H, Lang T, Brent B, Evans JA, Tibenderana JK, Crawley J, Russell EC, Levin M, Babiker AG, Gibb DM. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. - DOI - PubMed

