Insulin and insulin like growth factor II endocytosis and signaling via insulin receptor B
- PMID: 23497114
- PMCID: PMC3607927
- DOI: 10.1186/1478-811X-11-18
Insulin and insulin like growth factor II endocytosis and signaling via insulin receptor B
Abstract
Background: Insulin and insulin-like growth factors (IGFs) act on tetrameric tyrosine kinase receptors controlling essential functions including growth, metabolism, reproduction and longevity. The insulin receptor (IR) binds insulin and IGFs with different affinities triggering different cell responses.
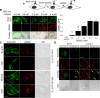
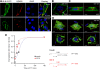
Results: We showed that IGF-II induces cell proliferation and gene transcription when IR-B is over-expressed. We combined biotinylated ligands with streptavidin conjugated quantum dots and visible fluorescent proteins to visualize the binding of IGF-II and insulin to IR-B and their ensuing internalization. By confocal microscopy and flow cytometry in living cells, we studied the internalization kinetic through the IR-B of both IGF-II, known to elicit proliferative responses, and insulin, a regulator of metabolism.
Conclusions: IGF-II promotes a faster internalization of IR-B than insulin. We propose that IGF-II differentially activates mitogenic responses through endosomes, while insulin-activated IR-B remains at the plasma membrane. This fact could facilitate the interaction with key effector molecules involved in metabolism regulation.
Figures

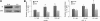
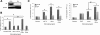
Similar articles
-
Ligand-mediated endocytosis and trafficking of the insulin-like growth factor receptor I and insulin receptor modulate receptor function.Front Endocrinol (Lausanne). 2014 Dec 17;5:220. doi: 10.3389/fendo.2014.00220. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25566192 Free PMC article. Review.
-
Ligand-Binding Affinity at the Insulin Receptor Isoform-A and Subsequent IR-A Tyrosine Phosphorylation Kinetics are Important Determinants of Mitogenic Biological Outcomes.Front Endocrinol (Lausanne). 2015 Jul 7;6:107. doi: 10.3389/fendo.2015.00107. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 26217307 Free PMC article.
-
Insulin and insulin-like growth factor II differentially regulate endocytic sorting and stability of insulin receptor isoform A.J Biol Chem. 2012 Mar 30;287(14):11422-36. doi: 10.1074/jbc.M111.252478. Epub 2012 Feb 8. J Biol Chem. 2012. PMID: 22318726 Free PMC article.
-
Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved.J Biol Chem. 2002 Oct 18;277(42):39684-95. doi: 10.1074/jbc.M202766200. Epub 2002 Jul 22. J Biol Chem. 2002. PMID: 12138094
-
Microarray analysis and identification of novel molecules involved in insulin-like growth factor-1 receptor signaling and gene expression.Recent Prog Horm Res. 2003;58:325-42. doi: 10.1210/rp.58.1.325. Recent Prog Horm Res. 2003. PMID: 12795426 Review.
Cited by
-
Pharmacologic manipulation of lysosomal enzyme transport across the blood-brain barrier.J Cereb Blood Flow Metab. 2016 Mar;36(3):476-86. doi: 10.1177/0271678X15614589. Epub 2015 Nov 3. J Cereb Blood Flow Metab. 2016. PMID: 26661222 Free PMC article.
-
Kinetics of Blood-Brain Barrier Transport of Monoclonal Antibodies Targeting the Insulin Receptor and the Transferrin Receptor.Pharmaceuticals (Basel). 2021 Dec 21;15(1):3. doi: 10.3390/ph15010003. Pharmaceuticals (Basel). 2021. PMID: 35056060 Free PMC article. Review.
-
Ligand-mediated endocytosis and trafficking of the insulin-like growth factor receptor I and insulin receptor modulate receptor function.Front Endocrinol (Lausanne). 2014 Dec 17;5:220. doi: 10.3389/fendo.2014.00220. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25566192 Free PMC article. Review.
-
Insulin Receptor Isoforms and Insulin Growth Factor-like Receptors: Implications in Cell Signaling, Carcinogenesis, and Chemoresistance.Int J Mol Sci. 2023 Oct 9;24(19):15006. doi: 10.3390/ijms241915006. Int J Mol Sci. 2023. PMID: 37834454 Free PMC article. Review.
-
EGF receptor lysosomal degradation is delayed in the cells stimulated with EGF-Quantum dot bioconjugate but earlier key events of endocytic degradative pathway are similar to that of native EGF.Oncotarget. 2017 Jul 4;8(27):44335-44350. doi: 10.18632/oncotarget.17873. Oncotarget. 2017. PMID: 28574831 Free PMC article.
References
-
- Efstratiadis A. Genetics of mouse growth. Int J Dev. Biol. 1998;42:955–976. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

