Optimizing heterologous protein production in the periplasm of E. coli by regulating gene expression levels
- PMID: 23497240
- PMCID: PMC3605120
- DOI: 10.1186/1475-2859-12-24
Optimizing heterologous protein production in the periplasm of E. coli by regulating gene expression levels
Abstract
Background: In Escherichia coli many heterologous proteins are produced in the periplasm. To direct these proteins to the periplasm, they are equipped with an N-terminal signal sequence so that they can traverse the cytoplasmic membrane via the protein-conducting Sec-translocon. For poorly understood reasons, the production of heterologous secretory proteins is often toxic to the cell thereby limiting yields. To gain insight into the mechanism(s) that underlie this toxicity we produced two secretory heterologous proteins, super folder green fluorescent protein and a single-chain variable antibody fragment, in the Lemo21(DE3) strain. In this strain, the expression intensity of the gene encoding the target protein can be precisely controlled.
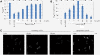
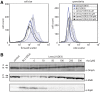
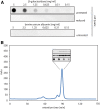
Results: Both SFGFP and the single-chain variable antibody fragment were equipped with a DsbA-derived signal sequence. Producing these proteins following different gene expression levels in Lemo21(DE3) allowed us to identify the optimal expression level for each target gene. Too high gene expression levels resulted in saturation of the Sec-translocon capacity as shown by hampered translocation of endogenous secretory proteins and a protein misfolding/aggregation problem in the cytoplasm. At the optimal gene expression levels, the negative effects of the production of the heterologous secretory proteins were minimized and yields in the periplasm were optimized.
Conclusions: Saturating the Sec-translocon capacity can be a major bottleneck hampering heterologous protein production in the periplasm. This bottleneck can be alleviated by harmonizing expression levels of the genes encoding the heterologous secretory proteins with the Sec-translocon capacity. Mechanistic insight into the production of proteins in the periplasm is key to optimizing yields in this compartment.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

