TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity
- PMID: 23497345
- PMCID: PMC3602024
- DOI: 10.1186/1744-8069-9-9
TRPV1, but not TRPA1, in primary sensory neurons contributes to cutaneous incision-mediated hypersensitivity
Abstract
Background: Mechanisms underlying postoperative pain remain poorly understood. In rodents, skin-only incisions induce mechanical and heat hypersensitivity similar to levels observed with skin plus deep incisions. Therefore, cutaneous injury might drive the majority of postoperative pain. TRPA1 and TRPV1 channels are known to mediate inflammatory and nerve injury pain, making them key targets for pain therapeutics. These channels are also expressed extensively in cutaneous nerve fibers. Therefore, we investigated whether TRPA1 and TRPV1 contribute to mechanical and heat hypersensitivity following skin-only surgical incision.
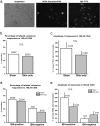
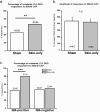
Results: Behavioral responses to mechanical and heat stimulation were compared between skin-incised and uninjured, sham control groups. Elevated mechanical responsiveness occurred 1 day post skin-incision regardless of genetic ablation or pharmacological inhibition of TRPA1. To determine whether functional changes in TRPA1 occur at the level of sensory neuron somata, we evaluated cytoplasmic calcium changes in sensory neurons isolated from ipsilateral lumbar 3-5 DRGs of skin-only incised and sham wild type (WT) mice during stimulation with the TRPA1 agonist cinnamaldehyde. There were no changes in the percentage of neurons responding to cinnamaldehyde or in their response amplitudes. Likewise, the subpopulation of DRG somata retrogradely labeled specifically from the incised region of the plantar hind paw showed no functional up-regulation of TRPA1 after skin-only incision. Next, we conducted behavior tests for heat sensitivity and found that heat hypersensitivity peaked at day 1 post skin-only incision. Skin incision-induced heat hypersensitivity was significantly decreased in TRPV1-deficient mice. In addition, we conducted calcium imaging with the TRPV1 agonist capsaicin. DRG neurons from WT mice exhibited sensitization to TRPV1 activation, as more neurons (66%) from skin-incised mice responded to capsaicin compared to controls (46%), and the sensitization occurred specifically in isolectin B4 (IB4)-positive neurons where 80% of incised neurons responded to capsaicin compared to just 44% of controls.
Conclusions: Our data suggest that enhanced TRPA1 function does not mediate the mechanical hypersensitivity that follows skin-only surgical incision. However, the heat hypersensitivity is dependent on TRPV1, and functional up-regulation of TRPV1 in IB4-binding DRG neurons may mediate the heat hypersensitivity after skin incision injury.
Figures


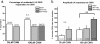


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

