Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia
- PMID: 23497456
- PMCID: PMC3623627
- DOI: 10.1186/1756-8722-6-21
Silvestrol exhibits significant in vivo and in vitro antileukemic activities and inhibits FLT3 and miR-155 expressions in acute myeloid leukemia
Abstract
Background: Activating mutations [internal tandem duplication (ITD)] or overexpression of the FMS-like tyrosine kinase receptor-3 (FLT3) gene are associated with poor outcome in acute myeloid leukemia (AML) patients, underscoring the need for novel therapeutic approaches. The natural product silvestrol has potent antitumor activity in several malignancies, but its therapeutic impact on distinct molecular high-risk AML subsets remains to be fully investigated. We examined here the preclinical activity of silvestrol in FLT3-ITD and FLT3 wild-type (wt) AML.
Methods: Silvestrol in vitro anti-leukemic activity was examined by colorimetric cell viability assay, colony-forming and flow cytometry assays assessing growth inhibition and apoptosis, respectively. Pharmacological activity of silvestrol on FLT3 mRNA translation, mRNA and protein expression was determined by RNA-immunoprecipitation, qRT-PCR and immunoblot analyses, respectively. Silvestrol in vivo efficacy was investigated using MV4-11 leukemia-engrafted mice.
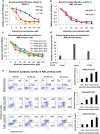
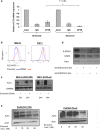
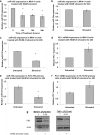
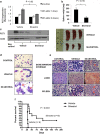
Results: Silvestrol shows antileukemia activity at nanomolar concentrations both in FLT3-wt overexpressing (THP-1) and FLT3-ITD (MV4-11) expressing AML cell lines (IC50 = 3.8 and 2.7 nM, respectively) and patients' primary blasts [IC50 = ~12 nM (FLT3-wt) and ~5 nM (FLT3-ITD)]. Silvestrol increased apoptosis (~4fold, P = 0.0001), and inhibited colony-formation (100%, P < 0.0001) in primary blasts. Silvestrol efficiently inhibited FLT3 translation reducing FLT3 protein expression by 80-90% and decreased miR-155 levels (~60%), a frequently co-regulated onco-miR in FLT3-ITD-positive AML. The median survival of silvestrol-treated vs vehicle-treated mice was 63 vs 29 days post-engraftment, respectively (P < 0.0001).
Conclusions: Silvestrol exhibits significant in vivo and in vitro antileukemic activities in AML through a novel mechanism resulting in inhibition of FLT3 and miR-155 expression. These encouraging results warrant a rapid translation of silvestrol for clinical testing in AML.
Figures
References
-
- Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, Holland KB, Wu YZ, Schwind S, Metzeler KH, Wen J, Baer MR, Powell BL, Carter TH, Kolitz JE, Wetzler M, Moore JO, Stone RM, Carroll AJ, Larson RA, Caligiuri MA, Marcucci G, Bloomfield CD. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010;116(18):3622–3626. doi: 10.1182/blood-2010-05-283648. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

