Noninvasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial
- PMID: 23497557
- PMCID: PMC3672522
- DOI: 10.1186/cc12549
Noninvasive ventilation immediately after extubation improves weaning outcome after acute respiratory failure: a randomized controlled trial
Abstract
Introduction: Noninvasive ventilation (NIV), as a weaning-facilitating strategy in predominantly chronic obstructive pulmonary disease (COPD) mechanically ventilated patients, is associated with reduced ventilator-associated pneumonia, total duration of mechanical ventilation, length of intensive care unit (ICU) and hospital stay, and mortality. However, this benefit after planned extubation in patients with acute respiratory failure of various etiologies remains to be elucidated. The aim of this study was to determine the efficacy of NIV applied immediately after planned extubation in contrast to oxygen mask (OM) in patients with acute respiratory failure (ARF).
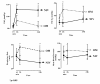
Methods: A randomized, prospective, controlled, unblinded clinical study in a single center of a 24-bed adult general ICU in a university hospital was carried out in a 12-month period. Included patients met extubation criteria with at least 72 hours of mechanical ventilation due to acute respiratory failure, after following the ICU weaning protocol. Patients were randomized immediately before elective extubation, being randomly allocated to one of the study groups: NIV or OM. We compared both groups regarding gas exchange 15 minutes, 2 hours, and 24 hours after extubation, reintubation rate after 48 hours, duration of mechanical ventilation, ICU length of stay, and hospital mortality.
Results: Forty patients were randomized to receive NIV (20 patients) or OM (20 patients) after the following extubation criteria were met: pressure support (PSV) of 7 cm H2O, positive end-expiratory pressure (PEEP) of 5 cm H2O, oxygen inspiratory fraction (FiO2)≤40%, arterial oxygen saturation (SaO2)≥90%, and ratio of respiratory rate and tidal volume in liters (f/TV)<105. Comparing the 20 patients (NIV) with the 18 patients (OM) that finished the study 48 hours after extubation, the rate of reintubation in NIV group was 5% and 39% in OM group (P=0.016). Relative risk for reintubation was 0.13 (CI=0.017 to 0.946). Absolute risk reduction for reintubation showed a decrease of 33.9%, and analysis of the number needed to treat was three. No difference was found in the length of ICU stay (P=0.681). Hospital mortality was zero in NIV group and 22.2% in OM group (P=0.041).
Conclusions: In this study population, NIV prevented 48 hours reintubation if applied immediately after elective extubation in patients with more than 3 days of ARF when compared with the OM group.
Trial registration number isrctn: 41524441.
Figures



References
-
- Keenan SP, Sinuff T, Burns KE, Muscedere J, Kutsogiannis J, Mehta S, Cook DJ, Ayas N, Adhikari NK, Hand L, Scales DC, Pagnotta R, Lazosky L, Rocker G, Dial S, Laupland K, Sanders K, Dodek P. Canadian Critical Care Trials Group/Canadian Critical Care Society Noninvasive Ventilation Guidelines Group. Clinical practice guidelines for the use of noninvasive positive-pressure ventilation and noninvasive continuous positive airway pressure in the acute care setting. CMAJ. 2011;183:E195–E214. - PMC - PubMed
-
- Burns KE, Adhikari NK, Keenan SP, Mead MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010;8:CD004127. Review. - PubMed
-
- Ferreyra G, Fanelli V, Del Sorbo L, Ranieri VM. Are guidelines for noninvasive ventilation during weaning still valid? Minerva Anestesiol. 2001;77:921–926. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

