Assessment of cigarette smoke particle deposition within the Vitrocell® exposure module using quartz crystal microbalances
- PMID: 23497606
- PMCID: PMC3635897
- DOI: 10.1186/1752-153X-7-50
Assessment of cigarette smoke particle deposition within the Vitrocell® exposure module using quartz crystal microbalances
Abstract
Background: Cigarette smoking is a cause of a variety of serious diseases, and to understand the toxicological impact of tobacco smoke in vitro, whole smoke exposure systems can be used. One of the main challenges of the different whole smoke exposure systems that are commercially available is that they dilute and deliver smoke in different ways, limiting/restricting the cross-comparison of biological responses. This is where dosimetry - dose quantification - can play a key role in data comparison. Quartz crystal microbalance (QCM) technology has been put forward as one such tool to quantify smoke particle deposition in vitro, in real-time.
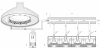
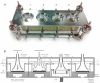
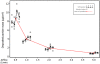
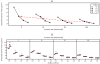
Results: Using four identical QCMs, installed into the Vitrocell® mammalian 6/4 CF Stainless exposure module, we were able to quantify deposited smoke particle deposition, generated and diluted by a Vitrocell® VC 10 Smoking Robot. At diluting airflows 0.5-4.0 L/min and vacuum flow rate 5 ml/min/well through the exposure module, mean particle deposition was in the range 8.65 ± 1.51 μg/cm(2)-0.72 ± 0.13 μg/cm(2). Additionally, the effect of varying vacuum flow rate on particle deposition was assessed from 5 ml/min/well - 100 ml/min/well. Mean deposited mass for all four airflows tested per vacuum decreased as vacuum rate was increased: mean deposition was 3.79, 2.75, 1.56 and 1.09 μg/cm(2) at vacuum rates of 5, 10, 50 and 100 ml/min/well respectively.
Conclusions: QCMs within the Vitrocell® exposure module have demonstrated applicability at defining particle dose ranges at various experimental conditions. This tool will prove useful for users of the Vitrocell® system for dose-response determination and QC purposes.
Keywords: Dosimetry; In vitro whole smoke exposure systems; Particle deposition; QCM; Quartz crystal microbalance; Tobacco smoke; Vitrocell®.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

