The three-dimensional structure of the biotin carboxylase-biotin carboxyl carrier protein complex of E. coli acetyl-CoA carboxylase
- PMID: 23499019
- PMCID: PMC3965354
- DOI: 10.1016/j.str.2013.02.001
The three-dimensional structure of the biotin carboxylase-biotin carboxyl carrier protein complex of E. coli acetyl-CoA carboxylase
Abstract
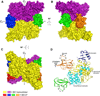
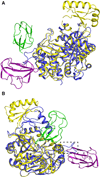
Acetyl-coenzyme A (acetyl-CoA) carboxylase is a biotin-dependent, multifunctional enzyme that catalyzes the regulated step in fatty acid synthesis. The Escherichia coli enzyme is composed of a homodimeric biotin carboxylase (BC), biotinylated biotin carboxyl carrier protein (BCCP), and an α2β2 heterotetrameric carboxyltransferase. This enzyme complex catalyzes two half-reactions to form malonyl-coenzyme A. BC and BCCP participate in the first half-reaction, whereas carboxyltransferase and BCCP are involved in the second. Three-dimensional structures have been reported for the individual subunits; however, the structural basis for how BCCP reacts with the carboxylase or transferase is unknown. Therefore, we report here the crystal structure of E. coli BCCP complexed with BC to a resolution of 2.49 Å. The protein-protein complex shows a unique quaternary structure and two distinct interfaces for each BCCP monomer. These BCCP binding sites are unique compared to phylogenetically related biotin-dependent carboxylases and therefore provide novel targets for developing antibiotics against bacterial acetyl-CoA carboxylase.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



References
-
- Alves J, Westling L, Peters EC, Harris JL, Trauger JW. Cloning, expression, and enzymatic activity of Acinetobacter baumannii and Klebsiella pneumoniae acetyl-coenzyme A carboxylases. Anal. Biochem. 2011;417:103–111. - PubMed
-
- Athappilly FK, Hendrickson WA. Structure of the biotinyl domain of acetyl-coenzyme A carboxylase determined by MAD phasing. Structure. 1995;3:1407–1419. - PubMed
-
- Bagautdinov B, Matsuura Y, Bagautdinova S, Kunishima N. Protein biotinylation visualized by a complex structure of biotin protein ligase with a substrate. J. Biol. Chem. 2008;283:14739–14750. - PubMed
-
- Bilder P, Lightle S, Bainbridge G, Ohren J, Finzel B, Sun F, Holley S, Al-Kassim L, Spessard C, Melnick M, et al. The structure of the carboxyltransferase component of acetyl-coA carboxylase reveals a zinc-binding motif unique to the bacterial enzyme. Biochemistry. 2006;45:1712–1722. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

