Morphine-enhanced apoptosis in selective brain regions of neonatal rats
- PMID: 23499314
- PMCID: PMC3640697
- DOI: 10.1016/j.ijdevneu.2013.02.009
Morphine-enhanced apoptosis in selective brain regions of neonatal rats
Abstract
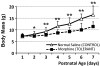
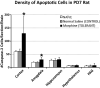
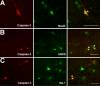
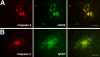
Prolonged neonatal opioid exposure has been associated with: antinociceptive tolerance, long-term neurodevelopmental delay, cognitive, and motor impairment. Morphine has also been shown to induce apoptotic cell death in vitro studies, but its in vivo effect in developing rat brain is unknown. Thus, we hypothesized that prolongued morphine administration in neonatal rats in a model of antinociceptive tolerance and dependence is associated with increased neuroapoptosis. We analyzed neonatal rats from the following groups (1) naïve group (n=6); (2) control group (normal saline (NS), n=5), and (3) morphine group (n=8). Morphine sulfate or equal volume of NS was injected subcutaneously twice daily for 6½ days starting on postnatal day (PD) 1. Development of antinociceptive tolerance was previously confirmed by Hot Plate test on the 7th day. Evidence of neuronal and glial apoptosis was determined by cleaved caspase-3 immunofluorescence combined with specific markers. At PD7, morphine administration after 6½ days significantly increased the density of apoptotic cells in the cortex and amygdala, but not in the hippocampus, hypothalamus, or periaqueductal gray. Apoptotic cells exhibited morphology analogous to neurons. Irrespective of the treatment, only a very few individual microglia but not astrocytes were caspase-3 positive. In summary, repeated morphine administration in neonatal rats (PD1-7) is associated with increased supraspinal apoptosis in distinct anatomical regions known to be important for sensory (cortex) and emotional memory processing (amygdala). Brain regions important for learning (hippocampus), and autonomic and nociceptive processing (hypothalamus and periaqueductal gray) were not affected. Lack of widespread glial apoptosis or robust glial activation following repeated morphine administration suggests that glia might not be affected by chronic morphine at this early age. Future studies should investigate long-term behavioral sequelae of demonstrated enhanced apoptosis associated with prolonged morphine administration in a neonatal rat model.
Copyright © 2013 ISDN. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch. Pediatr. Adolesc. Med. 2001;155:173–180. - PubMed
-
- Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr. Res. 2007;62:283–290. - PubMed
-
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, Boyle EM, Carbajal R, Bhutani VK, Moore MB, Kronsberg SS, Barton BA. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet. 2004;363:1673–1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

