Involvement of heparanase in atherosclerosis and other vessel wall pathologies
- PMID: 23499530
- PMCID: PMC3959806
- DOI: 10.1016/j.matbio.2013.03.002
Involvement of heparanase in atherosclerosis and other vessel wall pathologies
Abstract
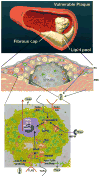
Heparanase, the sole mammalian endoglycosidase degrading heparan sulfate, is causally involved in cancer metastasis, angiogenesis, inflammation and kidney dysfunction. Despite the wide occurrence and impact of heparan sulfate proteoglycans in vascular biology, the significance of heparanase in vessel wall disorders is underestimated. Blood vessels are highly active structures whose morphology rapidly adapts to maintain vascular function under altered systemic and local conditions. In some pathologies (restenosis, thrombosis, atherosclerosis) this normally beneficial adaptation may be detrimental to overall function. Enzymatic dependent and independent effects of heparanase on arterial structure mechanics and repair closely regulate arterial compliance and neointimal proliferation following endovascular stenting. Additionally, heparanase promotes thrombosis after vascular injury and contributes to a pro-coagulant state in human carotid atherosclerosis. Importantly, heparanase is closely associated with development and progression of atherosclerotic plaques, including stable to unstable plaque transition. Consequently, heparanase levels are markedly increased in the plasma of patients with acute myocardial infarction. Noteworthy, heparanase activates macrophages, resulting in marked induction of cytokine expression associated with plaque progression towards vulnerability. Together, heparanase emerges as a regulator of vulnerable lesion development and potential target for therapeutic intervention in atherosclerosis and related vessel wall complications.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest: The authors declare that they have no conflict of interest.
Figures

References
-
- Aalkjaer C, Boedtkjer DB. Getting neointimal: the emergence of heparanase into the vascular matrix. Circulation Res. 2009;104:277–279. - PubMed
-
- Arvatz G, Shafat I, Levy-Adam F, Ilan N, Vlodavsky I. The heparanase system and tumor metastasis: is heparanase the seed and soil? Cancer Metastasis Rev. 2011;30:253–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

