Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone
- PMID: 23500068
- PMCID: PMC3658155
- DOI: 10.1016/j.preteyeres.2013.01.005
Adaptation of the central retina for high acuity vision: cones, the fovea and the avascular zone
Abstract
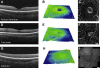
Presence of a fovea centralis is directly linked to molecular specification of an avascular area in central retina, before the fovea (or 'pit') begins to form. Modelling suggests that mechanical forces, generated within the eye, initiate formation of a pit within the avascular area, and its later remodelling in the postnatal period. Within the avascular area the retina is dominated by 'midget' circuitry, in which signals are transferred from a single cone to a single bipolar cell, then a single ganglion cell. Thus in inner, central retina there are relatively few lateral connections between neurons. This renders the region adaptable to tangential forces, that translocate of ganglion cells laterally/centrifugally, to form the fovea. Optical coherence tomography enables live imaging of the retina, and shows that there is greater variation in the morphology of foveae in humans than previously thought. This variation is associated with differences in size of the avascular area and appears to be genetically based, but can be modified by environmental factors, including prematurity. Even when the fovea is absent (foveal hypoplasia), cones in central retina adopt an elongated and narrow morphology, enabling them to pack more densely to increase the sampling rate, and to act as more effective waveguides. Given these findings, what then is the adaptive advantage of a fovea? We suggest that the advantages of having a pit in central retina are relatively few, and minor, but together work to enhance acuity.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures










References
-
- Abramov I, Gordon J, Hendrickson A, Hainline L, Dobson V, LaBossiere E. The retina of the newborn human infant. Science. 1982;217:265–7. - PubMed
-
- Akeo K, Shirai S, Okisaka S, Shimizu H, Miyata H, Kikuchi A, Nishikawa T, Suzumori K, Fujiwara T, Majima A. Histology of fetal eyes with oculocutaneous albinism. Arch Ophthalmol. 1996;114:613–6. - PubMed
-
- Akerblom H, Larsson E, Eriksson U, Holmstrom G. Central macular thickness is correlated with gestational age at birth in prematurely born children. Br J Ophthalmol. 2011;95:799–803. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

