Adipose tissue and adipocytes support tumorigenesis and metastasis
- PMID: 23500888
- PMCID: PMC3742583
- DOI: 10.1016/j.bbalip.2013.02.010
Adipose tissue and adipocytes support tumorigenesis and metastasis
Abstract
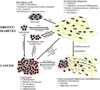
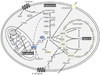
Adipose tissue influences tumor development in two major ways. First, obese individuals have a higher risk of developing certain cancers (endometrial, esophageal, and renal cell cancer). However, the risk of developing other cancers (melanoma, rectal, and ovarian) is not altered by body mass. In obesity, hypertrophied adipose tissue depots are characterized by a state of low grade inflammation. In this activated state, adipocytes and inflammatory cells secrete adipokines and cytokines which are known to promote tumor development. In addition, the adipocyte mediated conversion of androgens to estrogen specifically contributes to the development of endometrial cancer, which shows the greatest relative risk (6.3-fold) increase between lean and obese individuals. Second, many tumor types (gastric, breast, colon, renal, and ovarian) grow in the anatomical vicinity of adipose tissue. During their interaction with cancer cells, adipocytes dedifferentiate into pre-adipocytes or are reprogrammed into cancer-associated adipocytes (CAA). CAA secrete adipokines which stimulate the adhesion, migration, and invasion of tumor cells. Cancer cells and CAA also engage in a dynamic exchange of metabolites. Specifically, CAA release fatty acids through lipolysis which are then transferred to cancer cells and used for energy production through β-oxidation. The abundant availability of lipids from adipocytes in the tumor microenvironment, supports tumor progression and uncontrolled growth. Given that adipocytes are a major source of adipokines and energy for the cancer cell, understanding the mechanisms of metabolic symbiosis between cancer cells and adipocytes, should reveal new therapeutic possibilities. This article is part of a Special Issue entitled Lipid Metabolism in Cancer.
Keywords: Adipocytes; Cancer; Metabolic symbiosis; Metastasis; Obesity; Visceral adipose tissue.
Copyright © 2013 Elsevier B.V. All rights reserved.
Conflict of interest statement
None.
Figures

References
-
- Dirat B, Bochet L, Escourrou G, Valet P, Muller C. Unraveling the obesity and breast cancer links: A role for cancer-associated adipocytes. Endocr Dev. 2010;19:45–52. - PubMed
-
- Hanahan D, Coussens LM. Accessories to the crime: Functions of cell recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. - PubMed
-
- Martinez-Outschoorn UE, Whitaker-Menezes D, Pavlides S, Chiavarina B, Bonuccelli G, Trimmer C, Tsirigos A, Migneco G, Witkiewicz AK, Balliet R, Mercier I, Wang C, Flomenberg N, Howell A, Lin Z, Caro J, Pestell RG, Sotgia F, Lisanti MP. The autophagic tumor stroma model of cancer or "battery-operated tumor growth" a simple solution to the autophagy paradox. Cell Cycle. 2010;9:4297–4306. - PMC - PubMed
-
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS Journal. 2012;279:2610–2623. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

