Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1
- PMID: 23501697
- PMCID: PMC3767575
- DOI: 10.1161/CIRCRESAHA.111.300581
Apolipoprotein AI and high-density lipoprotein have anti-inflammatory effects on adipocytes via cholesterol transporters: ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1
Abstract
Rationale: Macrophage accumulation in adipose tissue associates with insulin resistance and increased cardiovascular disease risk. We previously have shown that generation of reactive oxygen species and monocyte chemotactic factors after exposure of adipocytes to saturated fatty acids, such as palmitate, occurs via translocation of NADPH oxidase 4 into lipid rafts (LRs). The anti-inflammatory effects of apolipoprotein AI (apoAI) and high-density lipoprotein (HDL) on macrophages and endothelial cells seem to occur via cholesterol depletion of LRs. However, little is known concerning anti-inflammatory effects of HDL and apoAI on adipocytes.
Objective: To determine whether apoAI and HDL inhibit inflammation in adipocytes and adipose tissue, and whether this is dependent on LRs.
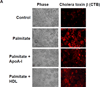
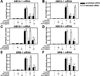
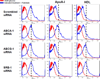
Methods and results: In 3T3L-1 adipocytes, apoAI, HDL, and methyl-β-cyclodextrin inhibited chemotactic factor expression. ApoAI and HDL also disrupted LRs, reduced plasma membrane cholesterol content, inhibited NADPH oxidase 4 translocation into LRs, and reduced palmitate-induced reactive oxygen species generation and monocyte chemotactic factor expression. Silencing ATP-binding cassette A-1 abrogated the effect of apoAI, but not HDL, whereas silencing ATP-binding cassette G-1 or scavenger receptor B-1 abrogated the effect of HDL but not apoAI. In vivo, apoAI transgenic mice fed a high-fat, high-sucrose, cholesterol-containing diet showed reduced chemotactic factor and proinflammatory cytokine expression and reduced macrophage accumulation in adipose tissue.
Conclusions: ApoAI and HDL have anti-inflammatory effects in adipocytes and adipose tissue similar to their effects in other cell types. These effects are consistent with disruption and removal of cholesterol from LRs, which are regulated by cholesterol transporters, such as ATP-binding cassette A-1, ATP-binding cassette G-1, and scavenger receptor B-1.
Keywords: ABC transporters; HDL; adipocytes; apolipoprotein AI; cholesterol.
Figures









Comment in
-
High-density lipoprotein extinguishes fire in fat.Circ Res. 2013 May 10;112(10):1304-6. doi: 10.1161/CIRCRESAHA.113.301382. Circ Res. 2013. PMID: 23661709 No abstract available.
References
-
- Murdolo G, Smith U. The dysregulated adipose tissue: A connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis. 2006;16(Suppl 1):S35–S38. - PubMed
-
- Mathieu P, Lemieux I, Despres JP. Obesity, inflammation, and cardiovascular risk. Clinical pharmacology and therapeutics. 2010;87:407–416. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

