C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence
- PMID: 23502316
- PMCID: PMC3781213
- DOI: 10.1038/ncb2698
C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence
Abstract
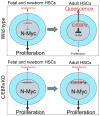
In blood, the transcription factor C/EBPa is essential for myeloid differentiation and has been implicated in regulating self-renewal of fetal liver haematopoietic stem cells (HSCs). However, its function in adult HSCs has remained unknown. Here, using an inducible knockout model we found that C/EBPa-deficient adult HSCs underwent a pronounced increase in number with enhanced proliferation, characteristics resembling fetal liver HSCs. Consistently, transcription profiling of C/EBPa-deficient HSCs revealed a gene expression program similar to fetal liver HSCs. Moreover, we observed that age-specific Cebpa expression correlated with its inhibitory effect on the HSC cell cycle. Mechanistically we identified N-Myc as a downstream target of C/EBPa, and loss of C/EBPa resulted in de-repression of N-Myc. Our data establish C/EBPa as a central determinant in the switch from fetal to adult HSCs.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

